658084-64-1
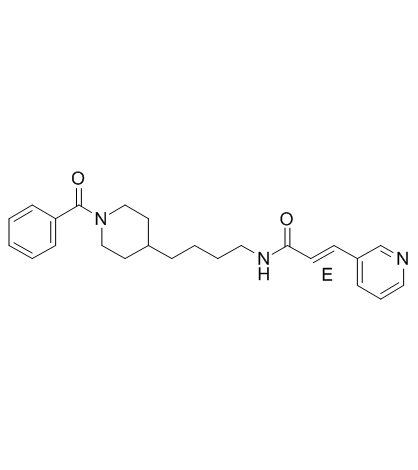
| Name | (E)-N-[4-(1-benzoylpiperidin-4-yl)butyl]-3-pyridin-3-ylprop-2-enamide |
|---|---|
| Synonyms |
2gvj
Daporinad (2E)-N-[4-(1-Benzoyl-4-piperidinyl)butyl]-3-(3-pyridinyl)acrylamide FK866 (APO866, Daporinad) FK 866 FK866 (APO866,Daporinad) APO866 (E)-N-[4-(1-BENZOYL-PIPERIDIN-4-YL)-BUTYL]-3-PYRIDIN-3-YL-ACRYLAMIDE FK866 |
| Description | FK866 is an effective inhibitor of nicotinamide phosphoribosyltransferase (NMPRTase) with an IC50 of 0.09 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.09 nM (NMPRTase) |
| In Vitro | Nampt inhibition with FK866 induces significant NAD+ intracellular reduction and selectively kills MM cells. FK866-induced cell death is associated with inhibition of Nampt activity, rather than protein expression, and higher NAD+ baseline levels in MM cells than normal PBMCs confer FK866 sensitivity. FK866 abrogates the survival advantage conferred by the bone marrow microenvironment[1]. FK866 prevents the [Ca2+]i increase induced by different mitogens and reduces the Ca2+ content of TG-responsive Ca2+ stores in Jurkat and in activated PBLs. FK866 reduces the Ca2+ content of TG-responsive Ca2+ stores in Jurkat cells but not in Bcl2-Jurkat cells[2]. Inhibition of NAMPT by FK866, or inhibition of SIRT by nicotinamide decreases proliferation and triggered death of 293T cells involving the p53 acetylation pathway[3]. |
| In Vivo | FK866 (30 mg/kg, i.p.) decreases the tumor burden in CB17-SCID mice, and the tumor tissue demonstrates a significant decrease in ERK phosphorylation and proteolytic cleavage of LC3[1]. |
| Cell Assay | MM1S cells (2×104 cells/well) are cultured for 72 and 96 hours in BMSC-coated 96-well plates in the presence or absence of drug. DNA synthesis is measured by (3H)-thymidine uptake, with (3H)-thymidine added (0.5 μCi/well) during the last 8 hours of cultures. |
| Animal Admin | CB17-SCID mice (28-35 days old) are irradiated (200 cGy), and then inoculated subcutaneously in the right flank with 3×106 MM1S cells in 100 μL RPMI 1640. After detection of tumor (2 weeks after the injection), 7 mice are treated intraperitoneally with either vehicle or FK866 (30 mg/kg body weight) twice a day for 4 days, repeated weekly over 3 weeks. Caliper measurements of the longest perpendicular tumor diameters are performed twice a week to estimate the tumor volume using the following formula: length×width2×0.5. Tumor growth inhibition (TGI) is calculated. Animals are killed when tumors reach 2 cm3 or the mice appear moribund. Survival is evaluated from the first day of treatment until death. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 629.9±51.0 °C at 760 mmHg |
| Molecular Formula | C24H29N3O2 |
| Molecular Weight | 391.506 |
| Flash Point | 334.8±30.4 °C |
| Exact Mass | 391.225983 |
| PSA | 62.30000 |
| LogP | 2.45 |
| Appearance | white to beige |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.589 |
| Storage condition | ?20°C |
| Water Solubility | DMSO: soluble10mg/mL, clear |