104987-12-4
| Name | ascomycin |
|---|---|
| Synonyms |
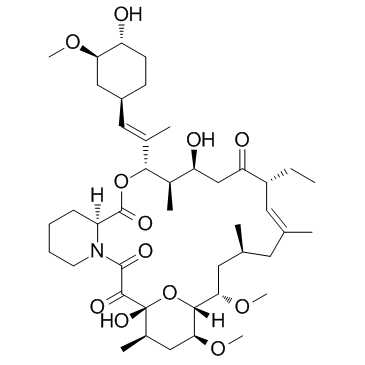
(1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-17-Ethyl-1,14-dihydroxy-12-{(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-propen-2-yl}-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatr ;icyclo[22.3.1.0]octacos-18-ene-2,3,10,16-tetrone
immunomycin 4H-Pyrrolo3,2-dpyrimidin-4-one,7-(2S,3S,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)-2-pyrrolidinyl-1,5-dihydro (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-8-ethyl-5,19-dihydroxy-3-{(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl}-14,16-dimethoxy-4,10,12,18-tetramethyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-3H-15,19-epoxypyrido[2,1-c][1,4]oxazacyclotricosine-1,7,20,21(4H,23H)-tetrone (1R,9S,12S,13R,14S,17R,18Z,21S,23S,24R,25S,27R)-17-Ethyl-1,14-dihydroxy-12-{(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-propen-2-yl}-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatr ;icyclo[22.3.1.0]octacos-18-ene-2,3,10,16-tetrone (1S)-1-(9-deazahypoxanthin-9-yl)-1,4-dideoxy-1,4-iMino-D-ribitol,iMMucillin H 7-((2S,3S,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)pyrrolidin-2-yl)-3H-pyrrolo[3,2-d]pyrimidin-4(5H)-one Ascomycin changchuanmycin (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-17-Ethyl-1,14-dihydroxy-12-{(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl}-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.0]octacos-18-ene-2,3,10,16-tetrone 7-[(2S,3S,4R,5R)-3,4-Dihydroxy-5-(hydroxymethyl)-2-pyrrolidinyl]-3,5-dihydro-4H-pyrrolo[3,2-d]pyrimidin-4-one (1S)-1-(9-deazahypoxanthin-9-yl)-1,4-dideoxy-1,4-imino-D-ribitol Isopamphos Immucillin-H 1,4-dideoxy-(1S)-1-C-(4-hydroxypyrrolo[3,2-d]pyrimidin-7-yl)-1,4-imino-D-ribitol MFCD00467131 Fodosine |
| Description | Ascomycin(Immunomycin, FR-900520, FK520) is an ethyl analog of tacrolimus (FK506) with strong immunosuppressant properties. IC50 Value: 0.55 nM [1]Target: in vitro: When we used either CD4+CD8+ thymocytes or peripheral T cells activated by phorbol ester and ionomycin, the cell surface induction of CD5 was also partially blocked by CsA, FK-520 and rapamycin [2]. Ascomycinalso had a 3-fold lower immunosuppressive potency in a popliteal lymph node hyperplasia assay, resulting in an equivalent therapeutic index consistent with a common mechanistic dependence on calcineurin inhibition [3].in vivo: In 14-day studies, nephrotoxicity was not induced by continuous i.p. infusion of ascomycin at 10 mg/kg/day or daily oral administration (up to 50 mg/kg/day) in rats on a normal diet, nor by continuous i.v. infusion (up to 6 mg/kg/day) in rats on a low salt diet to enhance susceptibility [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 868.3±75.0 °C at 760 mmHg |
| Melting Point | 153-157ºC |
| Molecular Formula | C43H69NO12 |
| Molecular Weight | 792.008 |
| Flash Point | 478.9±37.1 °C |
| Exact Mass | 791.481995 |
| PSA | 178.36000 |
| LogP | 3.81 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.546 |
| Storage condition | −20°C |
| Water Solubility | Soluble in DMSO at 65mg/ml; soluble in ethanol at 50mg/m; with warming. Very poorly soluble in water |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302 + H332-H319 |
| Precautionary Statements | P210-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | 36/37 |
| RIDADR | UN 1648 3 / PGII |
| WGK Germany | 3 |
| RTECS | KD4185000 |
| HS Code | 29419090 |