184582-62-5
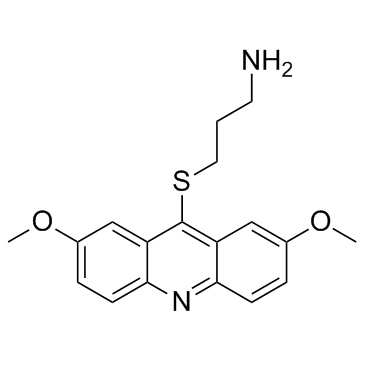
| Name | 3-((2,7-dimethoxyacridin-9-yl)thio)propan-1-amine |
|---|---|
| Synonyms |
3-[(2,7-Dimethoxy-9-acridinyl)sulfanyl]-1-propanamine
ldn-192960 |
| Description | LDN-192960 is a potent Haspin (Haploid Germ Cell-Specific Nuclear Protein Kinase) inhibitor with an IC50 of 0.010 µM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.010 μM (Haspin)[1] |
| In Vitro | A comparison of the profiles of LDN-192960 (compound 3) and 42 suggests that only six kinases, including haspin, are inhibited by both compounds ≥90% at 10 µM[1]. LDN-192960 alone does not cause detectable mitotic exit in the mitotic (phospho)-protein monoclonal-2 (MPM-2) assay in HeLa cells. In the presence of 1 µM ZM447439, even concentrations as low as 0.1 µM LDN-192960 cause substantial loss of MPM-2 epitopes, and similar results are obtained in the presence of Hesperadin. However, 10 µM LDN-192960 does not cause mitotic exit in combination with Aurora B inhibition, which is consistent with off-target effects at this higher dose[2]. |
| Cell Assay | U2OS cells and H2B-mRFP are used. For imaging, mitotic cells arrested in 5 µM nocodazole for ~8 h are harvested by “shake-off” and replated in 10% FBS, 25 mM Hepes, and phenol red-free DME containing 5 µM nocodazole in a 35-mm single chamber or 35-mm 4-chamber glass-bottom dishes coated with poly-d-lysine. Time-lapse confocal fluorescence imaging is performed using an inverted microscope. Immediately after kinase inhibitor (including LDN-192960) addition, two-color z stacks with 0.8 µm steps are collected with a 100-nm pinhole every 5 min for 15 h[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 536.1±40.0 °C at 760 mmHg |
| Molecular Formula | C18H20N2O2S |
| Molecular Weight | 328.429 |
| Flash Point | 278.0±27.3 °C |
| Exact Mass | 328.124542 |
| PSA | 82.67000 |
| LogP | 3.52 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.672 |
| Hazard Codes | Xi |
|---|