79952-42-4
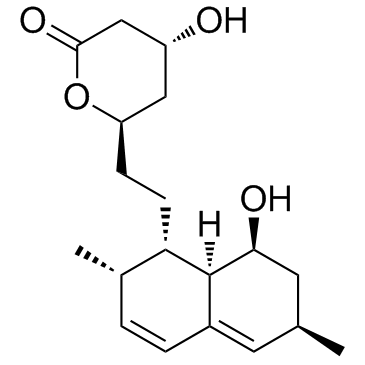
| Name | monacolin J |
|---|---|
| Synonyms |
Antibiotic MB 530A
(4R,6R)-4-Hydroxy-6-{2-[(1S,2S,6R,8S,8aR)-8-hydroxy-2,6-dimethyl-1,2,6,7,8,8a-hexahydro-1-naphthalenyl]ethyl}tetrahydro-2H-pyran-2-one Monacolin J (4R,6R)-6-[2-[(1S,2S,6R,8S,8aR)-8-hydroxy-2,6-dimethyl-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]ethyl]-4-hydroxyoxan-2-one Lovastatin diol lactone Simvastatin EP Impurity H |
| Description | Monacolin J is an inhibitor of cholesterol biosynthesis, and inhibits the activity of HMG-CoA reductase. |
|---|---|
| Related Catalog | |
| Target |
HMG-CoA reductase[1] |
| In Vitro | Monacolin J is an inhibitor of cholesterol biosynthesis, and inhibits the activity of HMG-CoA reductase. Na salt of Monacolin J causes 50% inhibition on reductase at 1.2 μg/mL[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 535.7±50.0 °C at 760 mmHg |
| Melting Point | 104-114 °C |
| Molecular Formula | C19H28O4 |
| Molecular Weight | 320.423 |
| Flash Point | 191.2±23.6 °C |
| Exact Mass | 320.198761 |
| PSA | 66.76000 |
| LogP | 1.81 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.558 |
| Hazard Codes | Xi |
|---|