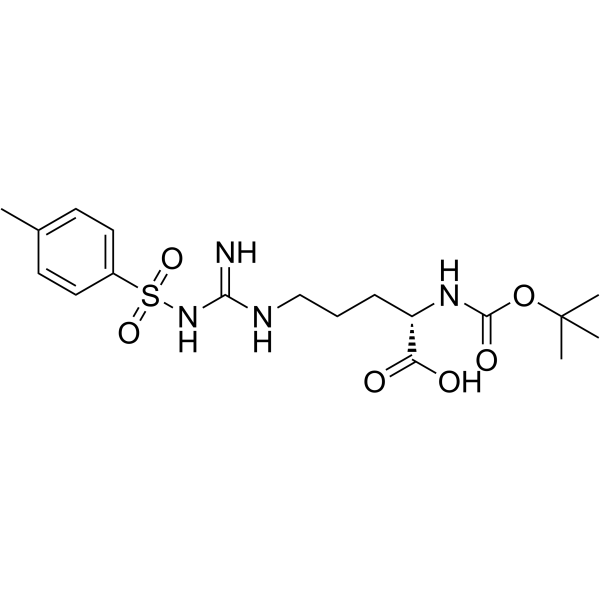
103429-32-9
| Name | ctap |
|---|---|
| Synonyms |
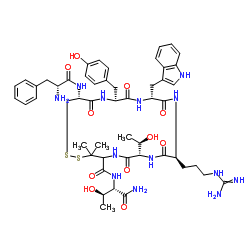
CYS2,TYR3,ARG5,PEN7-AMIDE
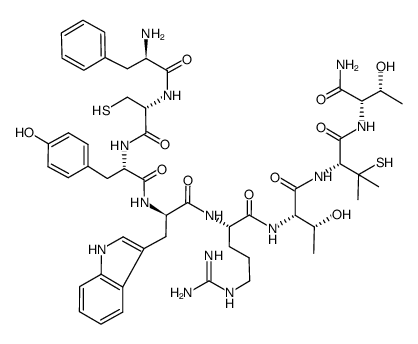
D-Phe-L-Cys-L-Tyr-D-Trp-L-Arg-L-Thr-L-Pen-L-Thr-NH2 M.W. 1104.30 C51H69N13O11S2 cyclic D-Pen-Cys-Tyr-D-Trp-Arg-ThrPen-Thr-NH2 H-D-PHE-CYS-TYR-D-TRP-ARG-THR-PEN-THR-NH2 (7S,10S,13R,16S,19R)-N-[(2S,3R)-1-Amino-3-hydroxy-1-oxo-2-butanyl]-10-(3-carbamimidamidopropyl)-16-(4-hydroxybenzyl)-7-[(1R)-1-hydroxyethyl]-13-(1H-indol-3-ylmethyl)-3,3-dimethyl-6,9,12,15,18-pentaoxo-19-(D-phenylalanylamino)-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carboxamide (4R,7S,10S,13R,16S,19R)-N-[(2S,3R)-1-Amino-3-hydroxy-1-oxo-2-butanyl]-10-(3-carbamimidamidopropyl)-16-(4-hydroxybenzyl)-7-[(1R)-1-hydroxyethyl]-13-(1H-indol-3-ylmethyl)-3,3-dimethyl-6,9,12,15,18-pentaoxo-19-(D-phenylalanylamino)-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carboxamide D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 |
| Description | CTAP is a potent, highly selective, and brain penetrant μ opioid receptor antagonist (IC50=3.5 nM) and displays over 1200-fold selectivity over δ opioid (IC50=4500 nM) and somatostatin receptors. CTAP can be used for the study of L-DOPA-induced dyskinesia (LID)[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 3.5 nM (μ opioid receptor) IC50: 4500 nM (δ opioid receptor)[1] |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Molecular Formula | C51H69N13O11S2 |
| Molecular Weight | 1104.304 |
| Exact Mass | 1103.468140 |
| PSA | 461.79000 |
| LogP | 0.44 |
| Index of Refraction | 1.699 |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3.0 |
|
~% 
103429-32-9 |
| Literature: Journal of Medicinal Chemistry, , vol. 29, # 11 p. 2370 - 2375 |
|
~% 
103429-32-9 |
| Literature: Journal of Medicinal Chemistry, , vol. 43, # 4 p. 569 - 580 |
|
~% 
103429-32-9 |
| Literature: Journal of Medicinal Chemistry, , vol. 43, # 4 p. 569 - 580 |
|
~% 
103429-32-9 |
| Literature: Journal of Medicinal Chemistry, , vol. 43, # 4 p. 569 - 580 |
|
~% 
103429-32-9 |
| Literature: Journal of Medicinal Chemistry, , vol. 43, # 4 p. 569 - 580 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |