8048-52-0
| Name | acriflavine |
|---|---|
| Synonyms |
ACRIFLAVINE
EUFLAVINE 3,6-Diamino-10-methylacridinium chloride - 3,6-acridinediamine (1:1:1) 3,6-Diamino-10-methylacridinium Chloride mixt with 3,6-Acridine Diamine 3,6-Diamino-10-methylacridinium chloride 3,6-acridinediamine (1:1:1) diacrid 3,6-Diamino-10-methylacridinium chloride 3,6-acridinediamine (1:1) flavipin Trypaflavine Acridinium, 3,6-diamino-10-methyl-, chloride, mixt. with 3,6-acridinediamine gonacin 3,6-Diamino-10-methylacridinium chloride - acridine-3,6-diamine (1:1:1) flavine Neutroflavine EUFLAVIN angiflan Neutral Acriflavine flavisept isravin MFCD00064307 |
| Description | Acriflavine is a fluorescent dye for labeling high molecular weight RNA. It is also a topical antiseptic. |
|---|---|
| Related Catalog | |
| In Vitro | Acriflavine is identified as a potent inhibitor of the MCT4 that can inhibit the binding between Basigin and MCT4. Acriflavine significantly inhibits growth and self-renewal potential of several glioblastoma neurosphere lines[1]. The HIF-1 inhibitor acriflavine decreases survival and growth of CML cells. It targets stem cell potential of CML cells[2]. |
| In Vivo | Acriflavine treatment inhibits intratumoral expression of VEGF and tumor vascularization[1]. In a murine CML model, acriflavine decreases leukemia development and reduces LSC maintenance[2]. Acriflavine retards tumor growth in a murine model of breast cancer. The combination of sunitinib with acriflavine significantly decreases vascular endothelial growth factor and TGF-β expression and reduces tumor vasculature followed by increased intratumor necrosis and apoptosis[3]. |
| Animal Admin | Mice: CML mice are treated daily with acriflavine (8 mg/kg) or PBS via intraperitoneal injection, for 10 days starting from day 7 after bone marrow transplantation[2]. |
| References |
| Melting Point | 179-181 °C |
|---|---|
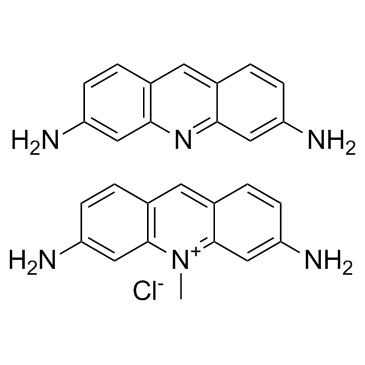
| Molecular Formula | C14H14ClN3 |
| Molecular Weight | 259.73 |
| PSA | 120.85000 |
| LogP | 3.86310 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful;N:Dangerousfortheenvironment; |
| Risk Phrases | R22;R37/38;R41;R50/53 |
| Safety Phrases | S26-S39-S60-S61 |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 3 |
| RTECS | AR9660000 |
| HS Code | 38249064 |
| HS Code | 38249064 |
|---|