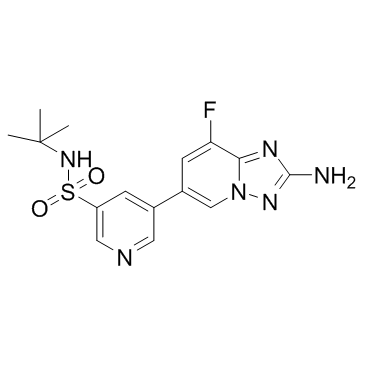
1159824-67-5
| Name | 5-(2-amino-8-fluoro-[1,2,4]triazolo[1,5-a]pyridin-6-yl)-N-tert-butylpyridine-3-sulfonamide |
|---|---|
| Synonyms |
cs-0710
fd5029 5-(2-Amino-8-fluoro[1,2,4]triazolo[1,5-a]pyridin-6-yl)-N-(2-methyl-2-propanyl)-3-pyridinesulfonamide CZC24832 |
| Description | CZC24832 is a highly selective and potent PI3Kγ inhibitor (IC50=27 nM) with apparent dissociation constants (Kdapp) of 19 nM. |
|---|---|
| Related Catalog | |
| Target |
PI3Kγ:27 nM (IC50) PI3Kβ:1.1 μM (IC50) PI3Kδ:8.194 μM (IC50) |
| In Vitro | CZC24832 is active in PI3Kγ-dependent cellular C5a-induced AKT Ser473 phosphorylation (IC50=1.2 μM) and N-formyl-methionine-leucinephenylalanine (fMLP)-induced neutrophil migration assays (IC50=1.0 μM)[1]. |
| In Vivo | CZC24832 shows suitable pharmacokinetic properties including low clearance (0.84 L per h per kg body weight) and high oral bioavailability (37%), thus allowing further characterization of the inhibitor in rodent models of inflammation. In an IL-8-dependent air pouch model, CZC24832 shows a dose-dependent reduction of granulocyte recruitment (80% inhibition at 10 mg per kg body weight) consistent with the degree of inhibition observed in PI3Kγ-null mice. Mice treated orally with 10 mg CZC24832 per kg body weight twice per day show a substantial decrease of bone and cartilage destruction (53% reduction by histopathological analysis) as well as of overall clinical parameters (38% reduction)[1]. |
| Cell Assay | RAW264.7 or THP-1 cells are starved for 2.5 h in serum-free medium before CZC24832 (0.1, 1, 10 and 100 μM) incubation for 30 min at 37°C. RAW264.7 cells are then stimulated for 3 min with C5a at a concentration of 0.6 μM, and THP-1 cells are stimulated with either insulin (1 uM, 10 min) or CSF (50 μg/mL, 5 min) at 37°C and lysed on ice. The detection of AKT phosphorylation (Ser473) is performed using the iBlot system[1]. |
| Animal Admin | Rats[1] Pharmacokinetics and oral bioavailability of CZC24832 are investigated in male Wistar rats following administration of a single intravenous (0.2 mg per kg body weight) or oral dose (10 mg per kg body weight). The dosing vehicle used is 0.5% (w/v) carboxymethyl cellulose in water for oral gavage. The intravenous dosing vehicle is 10% (v/v) DMSO in 30% (v/v) polyethylene glycol (PEG-400). Heparin blood for pharmacokinetic analysis is withdrawn retro-orbitally from mice or sublingually from rats to prepare plasma samples. These are homogenized with 10% (v/v) water and 3 volumes of acetonitrile and analyzed for CZC24832 by HPLC-MS/MS. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Molecular Formula | C15H17FN6O2S |
| Molecular Weight | 364.398 |
| Exact Mass | 364.111786 |
| PSA | 124.38000 |
| LogP | 1.27 |
| Index of Refraction | 1.687 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P280-P305 + P351 + P338-P337 + P313 |
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |