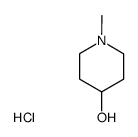
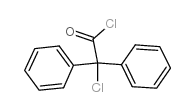
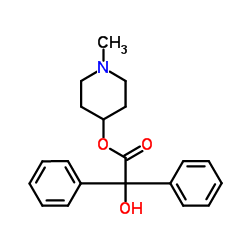
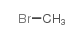5634-41-3
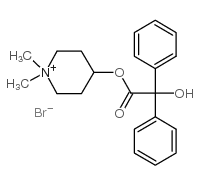
| Name | (1,1-dimethylpiperidin-1-ium-4-yl) 2-hydroxy-2,2-diphenylacetate,bromide |
|---|---|
| Synonyms |
Bromuro de parapenzolato
Bromure de parapenzolate Parapenzolate bromide Parapenzolati bromidum Parapenzolatbromid 4-benziloyloxy-1,1-dimethyl-piperidinium,bromide 4-[2-hydroxy(diphenyl)acetoxy]-1,1-dimethylpiperidinium bromide |
| Description | Parapenzolate bromide, an antispasmodic, is an orally active mAChR antagonist. Parapenzolate bromide is an anticholinergic agent[1][2]. |
|---|---|
| Related Catalog | |
| In Vivo | Parapenzolate bromide (10 mg/kg, p.o. or i.v.) shows a significant analgesic effect in mice[1]. Parapenzolate bromide (0.5 mg/kg, i.v.) exhibits a spasmolytic activity in isolated gall bladder from guinea pigs[2]. Animal Model: Mice[1] Dosage: 10 mg/kg Administration: Oral administration (p.o.) or intravenous injection (i.v.) Result: Prolonged the time until death in convulsive mice. Animal Model: Guinea pigs[2] Dosage: 0.5 mg/kg Administration: Intravenous injection (i.v.) Result: Antagonized contractions induced by acetylcholine (100 μg/kg, i.v.) |
| References |
| Molecular Formula | C21H26BrNO3 |
|---|---|
| Molecular Weight | 420.34000 |
| Exact Mass | 419.11000 |
| PSA | 46.53000 |
|
~% 
5634-41-3 |
| Literature: Coan et al. Journal of the American Chemical Society, 1956 , vol. 78, p. 3701 |
|
~% 
5634-41-3 |
| Literature: Coan et al. Journal of the American Chemical Society, 1956 , vol. 78, p. 3701 |
|
~% 
5634-41-3 |
| Literature: Coan et al. Journal of the American Chemical Society, 1956 , vol. 78, p. 3701 |
|
~% 
5634-41-3 |
| Literature: Coan et al. Journal of the American Chemical Society, 1956 , vol. 78, p. 3701 |
|
~% 
5634-41-3 |
| Literature: Coan et al. Journal of the American Chemical Society, 1956 , vol. 78, p. 3701 |