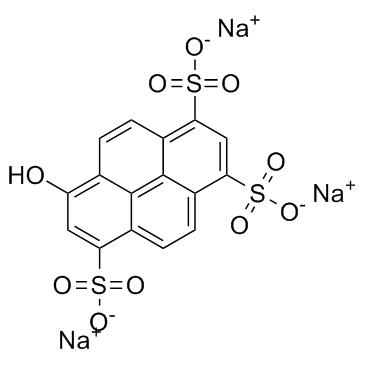
6358-69-6
| Name | pyranine |
|---|---|
| Synonyms |
Trisodium 8-hydroxy-1,3,6-pyrenetrisulfonate
Solvent Green 7 trisodium,8-hydroxypyrene-1,3,6-trisulfonate EINECS 228-783-6 1,3,6-Pyrenetrisulfonic acid, 8-hydroxy-, sodium salt (1:3) MFCD00037575 trisodium 8-hydroxypyrene-1,3,6-trisulfonate |
| Description | Pyranine as a new class of fluorescent chemosensor for the Cu+ ion is reported. |
|---|---|
| Related Catalog | |
| In Vitro | Pyranine is capable of discriminating ranges of cations from the Cu+ ion, even in competing environment. Pyranine displays a rapid fluorescence response (t1/2=1.66 min) towards the Cu+ ion, and the micromolar detection limit enables the detection of the ion in environmental samples. The observed stoichiometry of complexation between Pyranine and Cu+ is 2:1[1]. The pH-sensitive fluorescent indicator Pyranine is studied to determine its usefulness as probes for the living cornea and anterior chamber. Pyranine has properties which render it almost ideally suited for this purpose. Adequate concentrations are reached in the cornea and anterior chamber after topical administration; measurements can be made by fluorophotometry for many hours. The pH is calculated by measuring the ratio of fluorescent intensity at two excitation wavelengths, I463/I404, a measurement which is dependent on pH but independent of the concentration of the fluorophore and other variables which can alter the intensity. In the rabbit eye, Pyranine in the cornea and anterior chamber is observed to undergo easily measurable changes in fluorescent ratios associated with lid closure and contact lens wear, indicating its sensitivity to mild changes in pH[2]. Pyranine (Py) is a fluorescence probe[3]. |
| Cell Assay | PAAm-κC composite gels are prepared by free-radical copolymerization using 0.71 g of AAm, 0.01 g of BIS (N, N’-methylenebisacrylamide), 0.008 g of APS (ammonium persulfate) and 2 µl of TEMED (tetramethylethylenediamine) are dissolved in 5 mL of distilled water (pH 6.5) by heating. The heated mixture solution is held at 80°C. Then different amounts of κ-carrageenan (0.5, 1, 1.5, 2, 2.5 and 3 (w/v) % κC) are added. Pyranine (Py) is used in the PAAm–κC composites as a fluorescence probe, which is a derivative of pyrene possessing three SO3- groups, which can form bonds with positive charges on the gel. Pyranine (Py) concentration is kept constant at 4×10-4 M, for all experiments. The solution is stirred (200 rpm) for 15 min to achieve a homogenous sample solution. All samples are deoxygenated by bubbling nitrogen for 10 min just before the polymerization process. The drying and swelling experiments of disc-shaped PAAm–κC composite gels prepared are performed in air and in water, respectively, at various temperatures (30, 40, 50, and 60°C). A model LS-50 spectrometer equipped with a temperature controller is used for fluorescence intensity measurements, which are made at a 90° position and spectral bandwidths are kept at 5 nm. Disc-shaped gel samples are placed on the wall of a 1-cm path-length square quartz cell filled with air and/or water for the drying and swelling experiments[3]. |
| References |
| Density | 2.15 |
|---|---|
| Boiling Point | 171-178 °C10 mm Hg(lit.) |
| Melting Point | 62-63.5 °C(lit.) |
| Molecular Formula | C16H7Na3O10S3 |
| Molecular Weight | 524.385 |
| Flash Point | 250 °C |
| Exact Mass | 523.889465 |
| PSA | 216.97000 |
| LogP | 4.24430 |
| Water Solubility | 300 g/L (25 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S36/37-S37/39-S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UR2700000 |
| HS Code | 3204170000 |
|
~% 
6358-69-6 |
| Literature: Journal of the American Chemical Society, , vol. 106, # 26 p. 8086 - 8093 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2906299090 |
|---|---|
| Summary | 2906299090 other aromatic alcohols。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
![[3,6,8-tris(chlorosulfonyl)pyren-1-yl] acetate structure](https://image.chemsrc.com/caspic/460/91991-08-1.png)