123482-23-5
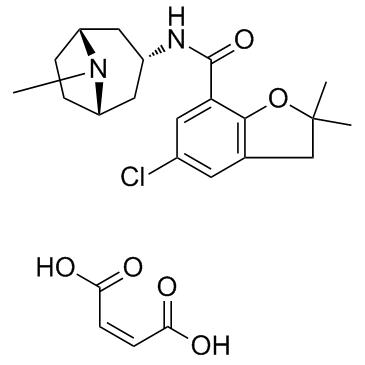
| Name | 5-Chloro-2,2-dimethyl-N-[(3-endo)-8-methyl-8-azabicyclo[3.2.1]oct -3-yl]-2,3-dihydro-1-benzofuran-7-carboxamide |
|---|---|
| Synonyms | Zatosetron (maleate) |
| Description | Zatosetron maleate is a potent and selective 5HT3 receptor antagonist. |
|---|---|
| Related Catalog | |
| Target |
5HT3 receptor[1] |
| In Vivo | Acute administration of 0.1 (n=21) and 0.3 (n=5) mg/kg Zatosetron maleate (Zatosetron) in male rats, but not 0.01, 0.05, 1.0 or 10 mg/kg (n=5, 3, 6 and 4, respectively) Zatosetron maleate or saline (n=5), leads to a significant reduction in the number of spontaneously active A10 dopamine cells. The number of spontaneously active A10 dopamine cells is not significantly different from 30 to 60 min post i.p. Zatosetron maleate (0.1 mg/kg) administration, shows a significant decrease by 60 to 90 min (0.65±0.11, P=0.03, n=5), a larger decrease by 90 to120 min (0.53±0.08, P=0.004, n=5) and remains at this significantly decreased level from 2 to 3 h (0.50+0.05, P=0.0004, n=5). Single-unit recordings show that Zatosetron maleate inhibits the activity of A10 dopamine cells following i.v. administration (ED50=0.12 mg/kg, n=8). Chronic administration of 0.1 mg/kg (n=16) Zatosetron maleate, but not 0.01, 1.0 or 10 mg/kg (n=4, 8 and 7, respectively) Zatosetron maleate or saline (n=5), leads to a significant reduction in the number of spontaneously active A10 dopamine cells[2]. |
| Animal Admin | Male rats are used and Zatosetron maleate (Zatosetron) is prepared as an aqueous solution. Acutely treated animals receive i.p. injections of Zatosetron maleate or saline 2 h before electrophysiological recordings; Chronically treated animals receive injections (i.p.) of Zatosetron maleate or saline once daily for 21 days, and receive their last injection 2 h before electrophysiological recordings. After completion of nine tracks, some animals are administered either apomorphine HCI (0.14 or 0.01 mg/kg i.v.) or haloperidol (0.1 mg/kg i.v.) and the number of dopamine cells is then counted in three additional tracks[2]. |
| References |
| Boiling Point | 435.1ºC at 760 mmHg |
|---|---|
| Molecular Formula | C23H29ClN2O6 |
| Molecular Weight | 464.94 |
| Flash Point | 216.9ºC |
| PSA | 45.06000 |
| LogP | 3.92120 |
| Vapour Pressure | 8.99E-08mmHg at 25°C |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|