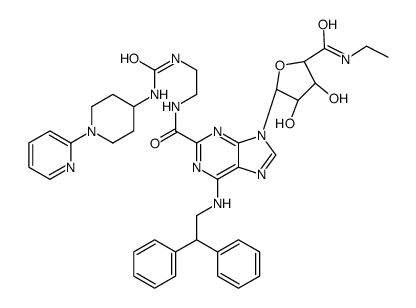
380221-63-6
| Name | 6-(2,2-diphenylethylamino)-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxyoxolan-2-yl]-N-[2-[(1-pyridin-2-ylpiperidin-4-yl)carbamoylamino]ethyl]purine-2-carboxamide |
|---|---|
| Synonyms |
3qak
6-[(2,2-Diphenylethyl)amino]-9-{(2R,3R,4S,5S)-5-[(ethylamino)carbonyl]-3,4-dihydroxytetrahydro-2-furanyl}-N-(2-[({[1-(2-pyridinyl)-4-piperidinyl]amino}carbonyl)amino]ethyl)-9H-purine-2-carboxamide UNII-8L3OAJ1R5A UK-432,097 6-(2,2-Diphenylethylamino)-9-[(2r,3r,4s,5s)-5-(Ethylcarbamoyl)-3,4-Dihydroxy-Oxolan-2-Yl]-N-[2-[(1-Pyridin-2-Ylpiperidin-4-Yl)carbamoylamino]ethyl]purine-2-Carboxamide |
| Description | UK-432097 is a highly potent and selective A2AAR agonist with a pKi of 8.4 for human A2AAR. UK-432097 has anti-inflammatory and anti-aggregatory properties. UK-432097 has the potential for COPD (Chronic Obstructive Pulmonary Disease) research[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
pKi: 8.4 (A2AAR)[1] |
| In Vitro | UK-432097 has an EC50 value of 0.66 nM using CHO cells expressing human WT A50AR[1]. UK-432097 (0.01, 0.1, 1, 10, 100, 1000 nM; pretreated for 2 hours) significantly increases cAMP accumulation in CHO-A2A cells[3]. |
| References |
| Molecular Formula | C40H47N11O6 |
|---|---|
| Molecular Weight | 777.87 |
| Exact Mass | 777.37100 |
| PSA | 227.76000 |
| LogP | 3.88310 |