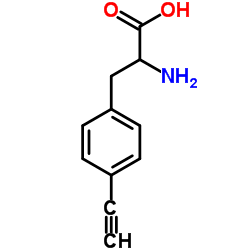
278605-15-5
| Name | (2S)-2-amino-3-(4-ethynylphenyl)propanoic acid |
|---|---|
| Synonyms |
L-Phenylalanine,4-ethynyl
p-ethynylphenylalanine 4-Ethynyl-L-phenylalanine HCl 4-Ethynylphenylalanine |
| Description | 4-Ethynyl-L-phenylalanine is a selective, reversible, potent and competitive inhibitor of tryptophan hydroxylase (TPH). 4-Ethynyl-L-phenylalanine is a competitive inhibitor with regard to the substrate tryptophan, with a Ki of 32.6 μM. 4-Ethynyl-L-phenylalanine selectively and reversibly inhibits the biosynthesis of serotonin[1]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 32.6 μM (TPH)[1] |
| In Vivo | 4-Ethynyl-L-phenylalanine (30 mg/kg; i.p.) decreases in 5-HT and 5-HIAA levels in the rat midbrain but not in tissue[1]. 4-Ethynyl-L-phenylalanine is not inhibiting the aromatic amino acid decarboxylase[1]. 4-Ethynyl-L-phenylalanine has a low affinity for various recombinant 5-HT receptors[1]. Animal Model: Male Sprague-Dawley rats (200 g)[1] Dosage: 30 mg/kg Administration: Intraperitoneal injection Result: Decreased in 5-HT and 5-HIAA levels in the rat midbrain. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 343.2±37.0 °C at 760 mmHg |
| Molecular Formula | C11H11NO2 |
| Molecular Weight | 189.210 |
| Flash Point | 161.4±26.5 °C |
| Exact Mass | 189.078979 |
| PSA | 63.32000 |
| LogP | 1.29 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.595 |
| Hazard Codes | Xi |
|---|