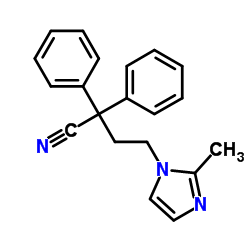
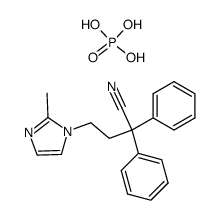
170105-16-5
| Name | 4-(2-methylimidazol-1-yl)-2,2-diphenylbutanamide |
|---|---|
| Synonyms |
Imidafenacin
[14C]-Imidafenacin 4-(2-Methyl-imidazol-1-yl)-2,2-diphenyl-butyramide Staybla 4-(2-methyl-1-imidazolyl)-2,2-diphenylbutyramide Imidafenacin (JAN/INN) 4-(2-Methyl-1H-imidazol-1-yl)-2,2-diphenylbutanamide Uritos (TN) Uritos Staybla (TN) |
| Description | Imidafenacin(KRP-197; ONO-8025) is a potent and selective inhibitor of M3 receptors with Kb of 0.317 nM; less potent for M2 receptors(IC50=4.13 nM).IC50 value: 0.3 nM(M3) [1]in vitro: KRP-197 showed equipotent anti-M2 and anti-M3 activity and decreased subtype-selectivity [1]. in vivo: Intraduodenal administration of KRP-197 (0.04±0.30 mg/kg) inhibited bladder contraction dose-dependently, and the ED30 value was 0.11 mg/kg. The inhibitory action of KRP-197 on the bladder contraction was 19 times as potent as that of oxybutynin. KRP-197 showed preventive action againstthe decrease in bladder capacity induced by carbachol(ED50 0.074 mg/kg, intragastric administration), andthe potency of the inhibitory action was 15-fold greaterthan that of oxybutynin [1]. The learning-inhibitory doses of intravenous oxybutynin hydrochloride and tolterodine tartrate were 0.3 and 3 mg/kg in sham-operated rats and 0.1 and 1 mg/kg in nbM-lesioned rats, respectively. Thus, the learning impairments by those antimuscarinics were more sensitive in nbM-lesioned rats than in sham-operated rats. On the other hand, intravenous administration of imidafenacin had no influence on learning in either case of the rats. In normal rats, however, intracerebroventricular administration of imidafenacin impaired learning to the same degree as that of oxybutynin hydrochloride [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 579.7±50.0 °C at 760 mmHg |
| Molecular Formula | C20H21N3O |
| Molecular Weight | 319.400 |
| Flash Point | 304.4±30.1 °C |
| Exact Mass | 319.168457 |
| PSA | 61.90000 |
| LogP | 2.42 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.603 |
| Storage condition | 2-8℃ |
|
~32% 
170105-16-5 |
| Literature: Kyorin Pharmaceutical Co., Ltd. Patent: US5932607 A1, 1999 ; |
|
~86% 
170105-16-5 |
| Literature: KYORIN PHARMACEUTICAL CO., LTD. Patent: EP1845091 A1, 2007 ; Location in patent: Page/Page column 4 ; EP 1845091 A1 |
|
~89% 
170105-16-5 |
| Literature: KYORIN PHARMACEUTICAL CO., LTD. Patent: EP1845091 A1, 2007 ; Location in patent: Page/Page column 5 ; EP 1845091 A1 |
|
~77% 
170105-16-5 |
| Literature: KYORIN PHARMACEUTICAL CO., LTD. Patent: EP1845091 A1, 2007 ; Location in patent: Page/Page column 6 ; EP 1845091 A1 |
|
~% 
170105-16-5 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 9, # 20 p. 3003 - 3008 |
|
~% 
170105-16-5 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 9, # 20 p. 3003 - 3008 |