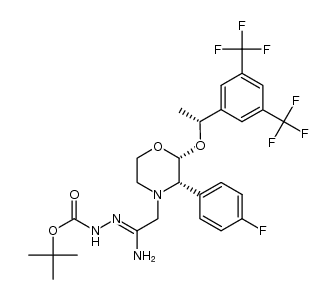
170729-80-3
| Name | Aprepitant |
|---|---|
| Synonyms |
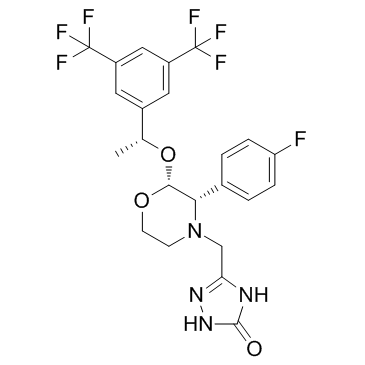
5-{[(2R,3S)-2-{(1R)-1-[3,5-Bis(trifluormethyl)phenyl]ethoxy}-3-(4-fluorphenyl)morpholin-4-yl]methyl}-1,2-dihydro-3H-1,2,4-triazol-3-on
5-[{(2R,3S)-2-((1R)-1-3,5-bis(trifluoro-methyl)phenylethoxy)-3-(4-fluorophenyl)-4-morpholinyl}methyl]-1,2-dihydro-3H-1,2,4-triazol-3-one 5-{[(2R,3S)-2-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethoxy}-3-(4-fluorophenyl)-4-morpholinyl]methyl}-1,2-dihydro-3H-1,2,4-triazol-3-one Emend aprepitant S1189_Selleck MK-0869 5-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)morpholin-4-yl]methyl]-1,2-dihydro-1,2,4-triazol-3-one MK-869 Emend (TN) 5-{[(2R,3S)-2-{(1R)-1-[3,5-bis(trifluorométhyl)phényl]éthoxy}-3-(4-fluorophényl)morpholin-4-yl]méthyl}-1,2-dihydro-3H-1,2,4-triazol-3-one 5-{[(2R,3S)-2-{(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy}-3-(4-fluorophenyl)morpholin-4-yl]methyl}-2,4-dihydro-3H-1,2,4-triazol-3-one [3H]-Aprepitant 5-{[(2R,3S)-2-{(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy}-3-(4-fluorophenyl)morpholin-4-yl]methyl}-1,2-dihydro-3H-1,2,4-triazol-3-one L-754030 Fosaprepitant Impurity 8 |
| Description | Aprepitant (MK-0869) is a selective and high-affinity neurokinin 1 receptor antagonist with a Kd of 86 pM. |
|---|---|
| Related Catalog | |
| Target |
Kd: 86 pM (Neurokinin 1 receptor)[1] |
| In Vitro | Aprepitant decreases the metabolic activity with an estimated IC50 value of 20 µM. Aprepitant induces cell-growth inhibition and G1 cell-cycle arrest. Aprepitant significantly induces apoptosis in Nalm-6 cells, and the apoptosis is mediated through caspase-3 activation. Aprepitant (20 µM) induces p53 accumulation and expression of pro-apoptotic p53 target genes[2]. Aprepitant (1, 5, 10 µM) inhibits HIV infection in MDM from both depressed and not depressed HIV negative individuals ex vivo in a dose-dependent manner. IC90 value of aprepitant is equivalent to 10 μM, and the IC50 value is about 5 μM[4]. |
| In Vivo | Aprepitant prevents the increase of NK-1R expression induced by in vivo NHP infection with B. burgdorferi. Aprepitant treatment prevents B. burgdorferi-induced increases in CCL2 protein levels in the CSF of NHPs. Aprepitant treatment prevents B. burgdorferi-induced increases in CCL2 and CXCL13 mRNA expression in the dorsal root ganglia of NHPs, prevents B. burgdorferi-induced increases in CCL2, CXCL13, IL-17A, and IL-6 mRNA expression in the spinal cord of NHPs. Aprepitant treatment attenuates B. burgdorferi infection-induced reductions in astrocyte activity/numbers[1]. Aprepitant (10 mg/kg, i.p.) significantly attenuates the CPP expression and locomotor activation produced by AMPH and cocaine in mice. In contrast, aprepitant significantly enhances the expression of CPP produced by morphine while significantly suppressing the locomotor activity of the mice conditioned with morphine. Aprepitant does not induce significant CPP or conditioned place aversion or locomotor activation or suppression[3]. Aprepitant (125 mg/day, p.o.) results in 1 log reduction in plasma levels of viral RNA as compared to non-treated controls[4]. |
| Cell Assay | The inhibitory effect of aprepitant on metabolic activity of Nalm-6 cells is assessed by uptake of thiazolyl blue tetrazolium bromide (MTT) by viable cells. Cells are plated onto 96-well plates at a density of 5000 cells/well. After treatment with aprepitant at 5, 10, 15, 20 and 30 µM for 24, 36 and 48 h, the cells are further incubated with 100 μL of MTT (0.5 mg/mL) at 37°C for 3 h. Untreated cells are defined as the control group. Following solubilization of precipitated formazan with 100 μL of DMSO, the optical densitometry is measured with an ELISA reader at a wavelength of 578 nm. |
| Animal Admin | Fifteen rhesus macaques are anesthetized and inoculated intrathecally with 1×108 live spirochetes into the cisterna magna, whereas five rhesus macaques are left uninfected and receive 1 mL of RPMI 1640 medium after removing an equivalent volume of CSF. The establishment of in vivo B. burgdorferi infection is confirmed by positive culture from at least necropsy tissue sample. The first set of animals are studied for 2 weeks and included two control animals (one of which is treated with aprepitant), two infected and untreated animals, and two infected animals that are treated with aprepitant. The second set of animals are studied for 4 weeks and included three control animals (one of which is treated with aprepitant), five infected and untreated animals, and four infected animals treated with aprepitant. Animals receive an average dose of aprepitant of 28 ± 6 mg/kg per day p.o. daily, and drug treatments are started 2 days before inoculation. These doses are consistent with standard veterinary regimens for the chosen drugs in NHP, and the 4-week duration of the study precludes the development of neural pathology that occurs at 8 weeks following B. burgdorferi infection. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Melting Point | 75-76 °C(lit.) |
| Molecular Formula | C23H21F7N4O3 |
| Molecular Weight | 534.427 |
| Exact Mass | 534.150208 |
| PSA | 83.24000 |
| LogP | 4.23 |
| Index of Refraction | 1.564 |
| Storage condition | -20°C Freezer |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Hazard Codes | Xn,Xi |
| Risk Phrases | R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 8 | |
|---|---|
| DownStream 0 | |



![(2R,3S)-2-[(1R)-1-[3,5-bis(Trifluoromethyl)phenyl)ethoxy]-3-(4-fluorophenyl)morpholine structure](https://image.chemsrc.com/caspic/069/171338-27-5.png)
![(R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol structure](https://image.chemsrc.com/caspic/464/368-63-8.png)