608141-41-9
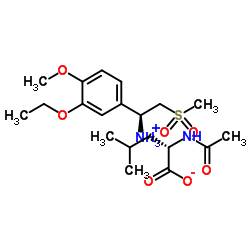
| Name | Apremilast |
|---|---|
| Synonyms |
QCR-202
(S)-N-(2-(1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)-1,3-dioxoisoindolin-4-yl)acetamide Apremilast N-{2-[(1S)-1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl}acetamide N-[2-[(1S)-1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-1,3-dioxoisoindol-4-yl]acetamide Otezla |
| Description | Apremilast is an orally available inhibitor of type-4 cyclic nucleotide phosphodiesterase (PDE-4) with an IC50 of 74 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 74 nM (PDE4)[1] |
| In Vitro | Apremilast inhibits TNF-α release by lipopolysaccharide (LPS) with an IC50 of 104 nM (pIC50=6.98±0.2), which almost exactly replicates previous reported TNF-α inhibition by Apremilast on peripheral blood mononuclear cells (PBMCs) (IC50=110 nM) and which is similar to the potency of Apremilast for PDE4 enzymatic inhibition (IC50=74 nM). These results are clearly consistent with the hypothesis that Apremilast inhibits TNF-α by increasing intracellular cAMP levels. PKA, Epac1 and Epac2 knockdowns prevented TNF-α inhibition and IL-10 stimulation by Apremilast[1]. |
| In Vivo | Apremilast, orally administered (5 mg/kg), significantly inhibits TNF-α production in the air pouch by 39 % (61±6 % of vehicle, P <0.001) and diminishes (by 28 %) the number of leukocytes present (72±12 % of vehicle, P<0.05). In agreement, immunohistologic analysis shows that neutrophil accumulation in the air pouch membrane is dramatically reduced by Apremilast. In the murine air pouch model, both Apremilast and methotrexate (MTX) significantly inhibit leukocyte infiltration, while Apremilast, but not MTX, significantly inhibits TNF-α release. The addition of MTX (1 mg/kg) to Apremilast (5 mg/kg) yields no more inhibition of leukocyte infiltration or TNF-α release than with Apremilast alone[1]. Apremilast is a novel, oral PDE4 inhibitor that has been shown to regulate inflammatory mediators. After oral administration of Apremilast, a mean maximum plasma concentration (Cmax) is found to be 67.00±14.87 ng/mL. The plasma concentration of Apremilast decreases rapidly and is eliminated from plasma with a terminal half-life of 0.92±0.46 h[2] |
| Cell Assay | Raw 264.7 cells (100,000) are grown in 96-well plates. After 24 h, cells are stimulated with vehicle (final concentration of 0.025% DMSO) or with Apremilast at the indicated concentrations. After 30 minutes cells are stimulated with LPS 1 μg/mL for 4 h. When studying CGS21680 , SCH58261, ZM241385, BAY60-6583, or GS6201, the adenosine receptor ligands are added 15 minutes before Apremilast. Methotrexate is added 24 h and 1 h before Apremilast. Supernates are then collected and TNF-α levels are quantified with the Mouse TNF-α Quantikine ELISA Kit. IC50 (EC50) calculations are made using non-linear regression, sigmoidal dose-response, constraining the top to 100 % and bottom to 0 %, allowing variable slope, using GraphPad Prism v6.00[1]. |
| Animal Admin | Mice[1] Male mice are given weekly intraperitoneal injections of either MTX (1 mg/kg) or vehicle (PBS) for 4 weeks. Air pouches are generated by subcutaneous injection of 3 mL of sterile air and reinflated with 1.5 mL of sterile air 2 days later. Vehicle (0.5 % carboxymethylcellulose and 0.25 % Tween 80) or Apremilast (5 mg/kg) are orally dosed, with a syringe through a blunt-ended curved feeding tube, 24 h and 1 h before inflammation is induced on day 6 by injection of 1 mL of 2 % carrageenan suspension. Four hours later, mice are killed by CO2 narcosis, and exudates harvested with 2 mL PBS. Leukocytes are counted in a hemocytometer chamber and concentrations of cytokines are measured by ELISA or by the Luminex platform. Rats[2] Male Sprague Dawley rats (180-220 g) are used to study the pharmacokinetics of Apremilast. Diet is prohibited for 12 h before the experiment, but water is freely available. Blood samples (0.3 mL) are collected from the tail vein into heparinized 1.5 mL polythene tubes at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8 and 12 h after oral administration of Apremilast (6.0 mg/kg). The samples are immediately centrifuged at 4,000 g for 8 min. The plasma obtained (100 µL) is stored at −20°C until analysis. Plasma Apremilast concentration versus time data for each rat is analyzed by DAS (Drug and statistics) software. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 741.3±60.0 °C at 760 mmHg |
| Molecular Formula | C22H24N2O7S |
| Molecular Weight | 460.500 |
| Flash Point | 402.1±32.9 °C |
| Exact Mass | 460.130432 |
| PSA | 130.95000 |
| LogP | 1.75 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.612 |
|
~75% 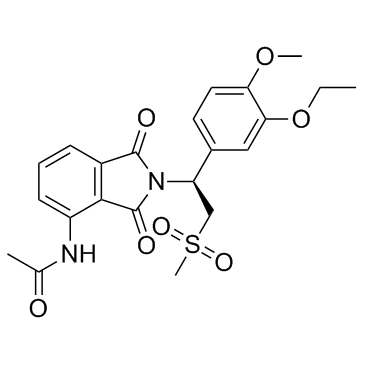
608141-41-9 |
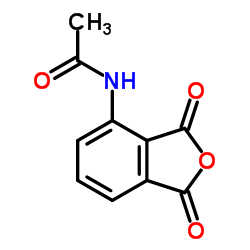
| Literature: CELGENE CORPORATION; ADAMS, Mary; STEVENS, Randall; SCHAFER, Peter, H. Patent: WO2014/74846 A1, 2014 ; Location in patent: Page/Page column 21 ; |
|
~75% 
608141-41-9 |
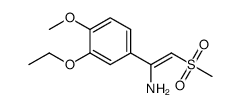
| Literature: Man, Hon-Wah; Schafer, Peter; Wong, Lu Min; Patterson, Rebecca T.; Corral, Laura G.; Raymon, Heather; Blease, Kate; Leisten, Jim; Shirley, Michael A.; Tang, Yang; Babusis, Darius M.; Chen, Roger; Stirling, Dave; Muller, George W. Journal of Medicinal Chemistry, 2009 , vol. 52, # 6 p. 1522 - 1524 |
|
~% 
608141-41-9 |
| Literature: US2014/81032 A1, ; |
|
~% 
608141-41-9 |
| Literature: US2014/81032 A1, ; |
|
~% 
608141-41-9 |
| Literature: WO2014/74846 A1, ; |
|
~% 
608141-41-9 |
| Literature: WO2014/74846 A1, ; |
| Precursor 6 | |
|---|---|
| DownStream 0 | |