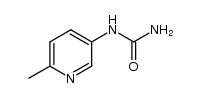
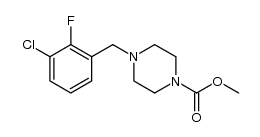
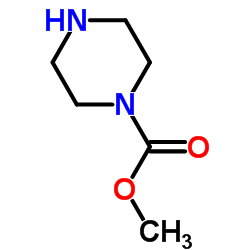
873697-71-3
| Name | methyl 4-[[2-fluoro-3-[(6-methylpyridin-3-yl)carbamoylamino]phenyl]methyl]piperazine-1-carboxylate |
|---|---|
| Synonyms |
methyl 4-(2-fluoro-5-(3-(6-methylpyridin-3-yl)ureido)benzyl)piperazine-1-carboxylate
omecamtiv mecarbil 1-Piperazinecarboxylic acid, 4-[[2-fluoro-3-[[[(6-methyl-3-pyridinyl)amino]carbonyl]amino]phenyl]methyl]-, methyl ester CK 1827452 Methyl 4-(2-fluoro-3-{[(6-methyl-3-pyridinyl)carbamoyl]amino}benzyl)-1-piperazinecarboxylate methyl 4-(2-fluoro-3-(3-(6-methylpyridin-3-yl)ureido)benzyl)piperazine-1-carboxylate S2623_Selleck methyl 4-[(2-fluoro-3-{[(6-methyl(3-pyridyl))amino]carbonylamino}phenyl)methyl]piperazinecarboxylate Methyl 4-[[2-fluoro-3-[N'-(6-methylpyridin-3-yl)ureido]phenyl]methyl]piperazine-1-carboxylate |
| Description | Omecamtiv mecarbil is a cardiac myosin activator. |
|---|---|
| Related Catalog | |
| In Vitro | Omecamtiv mecarbil (10 μM) reduces the maximal ATPase (kcat) 4.5-fold and dramatically reduces the actin concentration at which ATPase is half-maximal (KATPase) 30-fold. The Omecamtiv mecarbil-induced inhibition of the actin-activated ATPase is evaluated in a concentration-dependent manner to determine the EC50 (0.52 ± 0.10 μM). Omecamtiv mecarbil does not change the overall actin affinity. Omecamtiv mecarbil traps a population of myosin heads in a weak actin affinity state with slow product release. Omecamtiv mecarbil can reduce the actin sliding velocity more than 100-fold in the in vitro motility assay[3]. |
| In Vivo | Omecamtiv mecarbil (100-1000 ng/mL) demonstrates concentration-dependent increases in FS in Sprague−Dawley rats model. Omecamtiv mecarbil demonstrates good PK parameters in both rats (Sprague−Dawley) and dogs (Beagle) with clearances of 22 and 7.2 mL/min/kg, volumes of 3.5 and 3.6 L/kg, and bioavailabilities (F%) of 100 and 80%, respectively[1]. Omecamtiv mecarbil does not affect the phosphorylation status of myofilament proteins in both WT and KO hearts as shown by the absence of significant differences between pre and post Omecamtiv mecarbil samples within WT and KO groups, or affect the force generation at maximal Ca2+ activation (pCa 4.5) in any of the groups. Omecamtiv mecarbil increases the responsiveness of the cardiac myofilaments to Ca2+ at submaximal Ca2+-activations[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 456.8±45.0 °C at 760 mmHg |
| Melting Point | 180℃ |
| Molecular Formula | C20H24FN5O3 |
| Molecular Weight | 401.435 |
| Flash Point | 230.1±28.7 °C |
| Exact Mass | 401.186310 |
| PSA | 90.29000 |
| LogP | 1.36 |
| Appearance | white solid |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.639 |
| Storage condition | -20℃ |
|
~84% 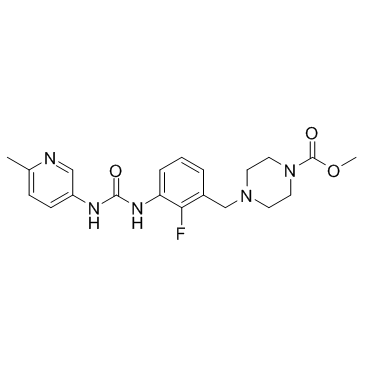
873697-71-3 |
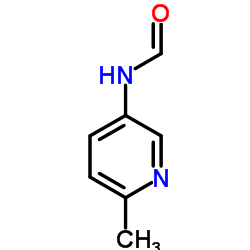
| Literature: CYTOKINETICS, INC. Patent: WO2007/78839 A2, 2007 ; Location in patent: Page/Page column 48 ; |
|
~70% 
873697-71-3 |
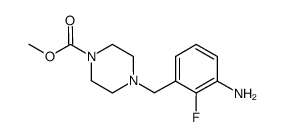
| Literature: Morgan, Bradley Paul; Muci, Alex; Lu, Pu-Ping; Kraynack, Erica Anne; Tochimoto, Todd; Morgans, David J. Patent: US2006/14761 A1, 2006 ; Location in patent: Page/Page column 33-34 ; |
|
~% 
873697-71-3 |
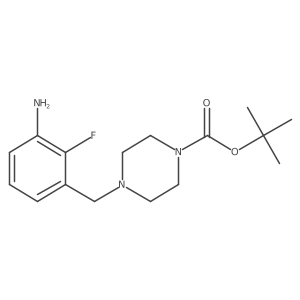
| Literature: Organic Letters, , vol. 13, # 12 p. 3262 - 3265 |
|
~% 
873697-71-3 |
| Literature: Breitler, Simon; Oldenhuis, Nathan J.; Fors, Brett P.; Buchwald, Stephen L. Organic Letters, 2011 , vol. 13, # 12 p. 3262 - 3265 |
|
~% 
873697-71-3 |
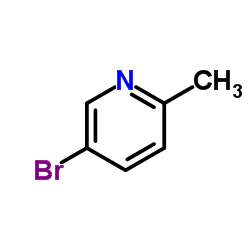
| Literature: Journal of the American Chemical Society, , vol. 134, # 27 p. 11132 - 11135 |
|
~% 
873697-71-3 |
| Literature: Journal of the American Chemical Society, , vol. 134, # 27 p. 11132 - 11135 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |