63074-08-8
| Name | Terazosin hydrochloride |
|---|---|
| Synonyms |
Terazosin
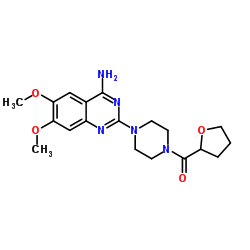
UNII:8L5014XET7 Vicard Itrin [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(oxolan-2-yl)methanone,hydrochloride Urodie [4-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl](tetrahydro-2-furanyl)methanone 6,7-bis(methyloxy)-2-[4-(tetrahydrofuran-2-ylcarbonyl)piperazin-1-yl]quinazolin-4-amine MFCD00467965 [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl](tetrahydrofuran-2-yl)methanone |
| Description | Terazosin hydrochloride is a quinazoline derivative and a competitive and orally active α1-adrenoceptor antagonist. Terazosin hydrochloride works by relaxing blood vessels and the opening of the bladder. Terazosin hydrochloride has the potential for benign prostatic hyperplasia (BPH) and high blood pressure treatment[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
α1-adrenoceptor[1] |
| In Vitro | Terazosin does not discriminate cloned α1-adrenoceptor subtypes transiently expressed in COS cells[1]. |
| In Vivo | Terazosin can be used to promote stone discharge in treatment of ureteral stones. Terazosin is reportedly safe and effective in treatment of distal ureteral stones, especially stones >5 mm[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 664.5±65.0 °C at 760 mmHg |
| Molecular Formula | C19H26ClN5O4 |
| Molecular Weight | 423.89 |
| Flash Point | 355.7±34.3 °C |
| PSA | 103.04000 |
| LogP | -0.96 |
| Appearance | powder,white to off-white |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.636 |
| Storage condition | Store at RT |
| Water Solubility | H2O: 25 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn,Xi |
|---|---|
| Risk Phrases | 22-36/37/38 |
| Safety Phrases | 26-36 |
| WGK Germany | 3 |
| RTECS | TK8044925 |