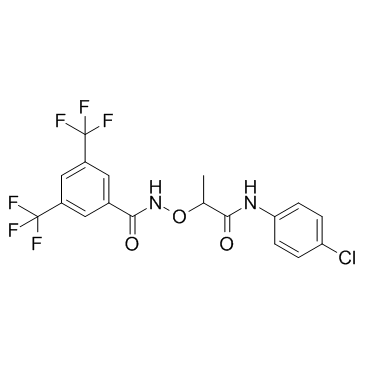
285986-88-1
| Name | N-[1-(4-chloroanilino)-1-oxopropan-2-yl]oxy-3,5-bis(trifluoromethyl)benzamide |
|---|---|
| Synonyms |
N-(2-(4-Chloroanilino)-1-methyl-2-oxoethoxy)-3,5-bis(trifluoromethyl)benzamide
CCG 1423 N-[2-[(4-Chlorophenyl)amino]-1-methyl-2-oxoethoxy]-3,5-bis(trifluoromethyl)-benzamide CCG-1423 N-({1-[(4-Chlorophenyl)amino]-1-oxo-2-propanyl}oxy)-3,5-bis(trifluoromethyl)benzamide |
| Description | CCG-1423 is a novel inhibitor of RhoA/C-mediated gene transcription that is capable of inhibiting invasion of PC-3 prostate cancer cells in a Matrigel model of metastasis.IC50 value: 1.5 uM [1]Target: Rho signaling inhibitorin vitro: CCG-1423 selectively inhibited spontaneous PC-3 prostate cancer cell invasion through a Matrigel matrix, but not the Gαi-dependent LPA-stimulated SKOV-3 ovarian cancer cell invasion, in vitro. At 100 μM, nearly complete inhibition of invasion was achieved with a lesser degree of toxicity than that induced by CCG-1423 at 10 μM [1]. SRF binds to this site in vivo and the SRF inhibitor CCG-1423 completely blocks STARS proximal reporter activity in H9c2 cells [3]. pharmacological MKL-inhibition with CCG-1423 significantly inhibited CCN1 promoter activity as well as mRNA and protein expression[4].in vivo: Pharmacological SRF inhibition by CCG-1423 reduced nuclear MKL1 and improved glucose uptake and tolerance in insulin-resistant mice in vivo [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Molecular Formula | C18H13ClF6N2O3 |
| Molecular Weight | 454.751 |
| Exact Mass | 454.051880 |
| PSA | 70.92000 |
| LogP | 6.59 |
| Appearance | white to beige |
| Index of Refraction | 1.525 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: soluble5mg/mL, clear (warmed) |
| Hazard Statements | H413 |
|---|---|
| RIDADR | NONH for all modes of transport |