38937-66-5
| Name | Suberoyl Bis-hydroxamic Acid |
|---|---|
| Synonyms |
octane-1,8-dihydroxamic acid
N,N'-Dihydroxyoctanediamide MFCD00192455 |
| Description | Suberoyl bis-hydroxamic acid (Suberohydroxamic acid; SBHA) is a competitive and cell-permeable HDAC1 and HDAC3 inhibitor with ID50 values of 0.25 μM and 0.30 μM, respectively[1].Suberoyl bis-hydroxamic acid renders MM cells susceptible to apoptosis and facilitates the mitochondrial apoptotic pathways[2].Suberoyl bis-hydroxamic acid can be used for the study of medullary thyroid carcinoma (MTC)[3]. |
|---|---|
| Related Catalog | |
| Target |
HDAC1:0.25 μM (IC50) HDAC3:0.30 μM (IC50) |
| In Vitro | Suberoyl bis-hydroxamic acid (10, 20 or 50 μM; 24 hours) combination with TRAIL improves apoptosis extent, and when TRAIL is combined with 20 μM SBHA (itself causing only 10–15% apoptosis), resulting in 45–50% cell death[1]. Suberoyl bis-hydroxamic acid (20-50 μM; 10-20 hours) alone has little effect on the expression of the proteins Bcl-xL, Mcl-1, and has albeit mildeffect on Bax. When it combines with TRAIL,which increases the ratio of relative protein expression of Bcl-xL and Bax in early periods, while the change in the ratio of Mcl-1 and Bax increases later in MM-BI and Ist-Mes2 cells[1]. Suberoyl bis-hydroxamic acid (30 μM; 6 hours) causes accumulation of acetylated histone H4 in MEL cells[2]. Apoptosis Analysis[1] Cell Line: MM-BI and Ist-Mes2 cells Concentration: 10 μM, 20 μM or 50 μM Incubation Time: 24 hours Result: Showed a cooperative effect in cell apoptosis. |
| In Vivo | Suberoyl bis-hydroxamic acid (intraperitoneal injection; 200 mg/kg; every 2 days; 12 days) reveals a marked increase in the active form of Notch1 (NICD) with a concomitant decrease in ASCL1. It reduces the MTC tumor growth[3]. Animal Model: Nude mice injected with human MTC cells[3] Dosage: 200 mg/kg Administration: Intraperitoneal injection; every 2 days; 12 days Result: Resulted in an average 55% inhibition of tumor growth in SBHA treatment group. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Melting Point | 153-155ºC(lit.) |
| Molecular Formula | C8H16N2O4 |
| Molecular Weight | 204.224 |
| Exact Mass | 204.111008 |
| PSA | 98.66000 |
| LogP | -1.81 |
| Appearance | lyophilized powder |
| Index of Refraction | 1.502 |
| Storage condition | −20°C |
| Water Solubility | H2O: soluble |
| Safety Phrases | 22-24/25 |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924199090 |
|
~92% 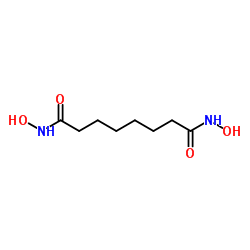
38937-66-5 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 21, # 13 p. 3866 - 3872 |
|
~% 
38937-66-5 |
| Literature: Journal of Chemical & Engineering Data, , vol. 29, # 3 p. 345 - 348 |
|
~% 
38937-66-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 53, # 8 p. 3038 - 3047 |
|
~% 
38937-66-5 |
| Literature: Synthetic Communications, , vol. 15, # 13 p. 1159 - 1164 |
|
~% 
38937-66-5 |
| Literature: Journal of the Chemical Society. Perkin Transactions 2, , vol. 1996, # 12 p. 2673 - 2679 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |