2756-87-8
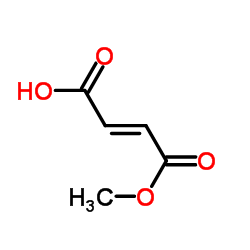
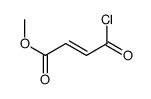
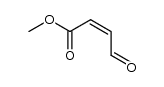
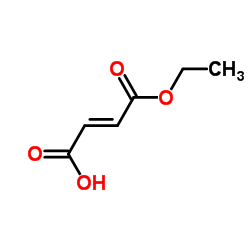
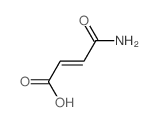
| Name | Fumaric Acid Monomethyl Ester |
|---|---|
| Synonyms |
MFCD00063174
(2E)-4-Methoxy-4-oxobut-2-enoic acid EINECS 220-412-6 (2E)-4-Methoxy-4-oxo-2-butenoic acid Methyl Hydrogen Fumarate 2-Butenedioic acid, monomethyl ester Monomethyl Fumarate 4-Methoxy-4-oxobut-2-enoic acid Fumaric acid, monomethyl ester |
| Description | Monomethyl fumarate, an active metabolite of Dimethyl fumarate (DMF), is a potent GPR109A agonist. Monomethyl fumarate has the potential for multiple neuroprotective pathways and other models of retinal disease[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Monomethyl fumarate completely inhibits forskolin induced cAMP synthesis with an IC50 of 70 nM. Monomethyl fumarate induces a dose-dependent Ca2+ signal in GPR109A transfected cells with an EC50 of 9.4 μM[1]. Monomethyl fumarate (25 μM; 24 hours) attenuates 7β-OHC-induced cytotoxicity: cell growth inhibition; decreased cell viability; mitochondrial dysfunction; and cell death induction[3]. |
| In Vivo | A single dose of Monomethyl fumarate (50-100 mg/kg; IP) before light exposure prevents these morphologic changes (bright light exposure induced photoreceptor death) in a dose-dependent manner[2]. Monomethyl fumarate (100 mg/kg) reduces retinal inflammation and oxidative stress. Monomethyl fumarate significantly suppresses light-induced retinopathy (LIR) upregulated genes in the NFkB pathway including: Nlrp3, Casp1, Il-1β, and Tnf-α[2]. Animal Model: Albino BALB/c mice (male, 6 weeks old)[2] Dosage: 50, 65, 75, 100 mg/kg Administration: IP Result: Prevented these morphologic changes (bright light exposure induced photoreceptor death) in a dose-dependent manner. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 250.0±23.0 °C at 760 mmHg |
| Melting Point | 144-145ºC |
| Molecular Formula | C5H6O4 |
| Molecular Weight | 130.099 |
| Flash Point | 108.9±16.1 °C |
| Exact Mass | 130.026611 |
| PSA | 63.60000 |
| LogP | -0.24 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.469 |
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H318 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R41 |
| Safety Phrases | S26-S39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2918990090 |
| Precursor 10 | |
|---|---|
| DownStream 5 | |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |