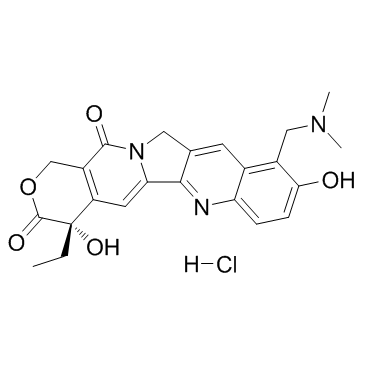
123948-87-8
| Name | topotecan |
|---|---|
| Synonyms |
MFCD00866235
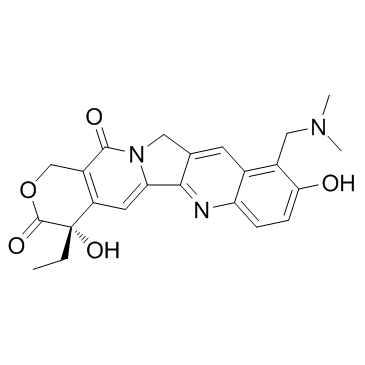
10-[(Dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione Topotecan hydrochloride hydrate 9-[(dimethylamino)methyl]-10-hydroxy-(20S)-camptothecin hydrochloride hydrate Topotecan EINECS 231-595-7 |
| Description | Topotecan (SKF 104864A; NSC 609669) is a Topoisomerase I inhibitor. The IC50 values of Topotecan at 24 h are 2.73±0.25 μM of U251 cells, 2.95±0.23 μM of U87 cells, 5.46±0.41 μM of GSCs-U251 and 5.95±0.24 μM of GSCs-U87. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I |
| In Vitro | Topotecan (SKF104864) obviously inhibits proliferation of not only human glioma cells but also glioma stem cells (GSCs) in a dose- and time-dependent manner. According to the IC50 values at 24 h, 3 μM of Topotecan (SKF104864) is selected as the optimal administration concentration. In addition, Topotecan (SKF104864) induces cell cycle arrest in G0/G1 and S phases and promoted apoptosis. Results show that the cell viability is inhibited by Topotecan (SKF104864) in a dose-dependent manner. 2, 20 and 40 μM of Topotecan obviously inhibits the cell viability compared with the control groups. The IC50 values of Topotecan (SKF104864) at 24 h are 2.73±0.25 μM of U251 cells, 2.95±0.23 μM of U87 cells, 5.46±0.41 μM of GSCs-U251 and 5.95±0.24 μM of GSCs-U87. Thus 3 μM of Topotecan is selected as the optimal administration concentration in the subsequent experiments[1]. |
| In Vivo | NUB-7 metastatic model, the animals belonging to all the 4 groups are sacrificed after 14 days treatment. Compared with the control, Low dose metronomic (LDM) Topotecan (TP) and TP+Pazopanib (PZ) liver weights are significantly lower in TP+PZ-treated animals, compared with PZ. Microscopic tumors are visible in the livers of mice belonging to all the groups except TP+PZ confirming the ability of TP+PZ to control liver metastasis. In a previous dose-response study, the daily dose of oral metronomic Topotecan (0.5, 1.0, and 1.5 mg/kg) causes greater reduction in microvascular density compared with weekly maximum-tolerated dose regimen (7.5 and 15 mg/kg) in an ovarian cancer model, but the mice treated with 1.5 mg/kg daily, oral Topotecan show decreased food intake, and a lesser antitumor effect[2]. |
| Cell Assay | The U251, U87, GSCs-U251 and GSCs-U87 cells are seeded at a density of 2×104 cells per well in 96-well plates separately, and incubated for 24 h. Cells are administered with Shikonin and Topotecan (0.02, 0.2, 2, 20, 40 μM). After the treatment, 10 μL of cell counting kit-8 (CCK-8) is added into each well for additional 1-hour incubation at 37°C. The optical density (OD) is read with a microplate reader at 450 nm[1]. |
| Animal Admin | Mice[2] For subcutaneous xenograft studies, we used SK-N-BE, SH-SY5Y, KHOS, and RH30. 1×106 cells are implanted subcutaneously into the inguinal fat pad of each of nonobese diabetic/severe combined immune deficient (NOD/SCID) mice. When tumors reached 0.5 cm in diameter, the animals are randomized into 4 groups and treated daily by oral gavage. The animals are grouped as: Control group, LDM Topotecan group or LDM TP (1 mg/kg Topotecan), Pazopanib group or PZ (150 mg/kg Pazopanib) and combination group or TP+PZ (1 mg/kg Topotecan+150 mg/kg Pazopanib). To compare pulse Topotecan with LDM TP in KHOS osteosarcoma model, PZ is replaced by weekly oral dose of pulse Topotecan (SKF104864) or Pulse TP (15 mg/kg Topotecan (SKF104864)). The criteria for endpoint are tumor sizes exceeding 2.0 cm in diameter or animals showing signs of morbidity. The tumor sizes are measured on a daily basis until the endpoint or sacrifice. The long (D) and short diameters (d) are measured with calipers. Tumor volume (cm3) is calculated as V=0.5×D×d2. When the endpoint is reached or at the end of the treatment, the animals are sacrificed by cervical dislocation. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 782.9±60.0 °C at 760 mmHg |
| Melting Point | −114 °C(lit.) |
| Molecular Formula | C23H23N3O5 |
| Molecular Weight | 421.446 |
| Flash Point | 427.3±32.9 °C |
| Exact Mass | 421.163757 |
| PSA | 104.89000 |
| LogP | 1.08 |
| Vapour density | 1.3 (vs air) |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.734 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: soluble |
| Hazard Codes | T,C,F,Xi |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S45-S36/37/39 |
| RIDADR | UN 3286 3/PG 2 |
| WGK Germany | 2 |
| RTECS | MW4025000 |
| Packaging Group | II |
| Hazard Class | 8 |
| HS Code | 2942000000 |
|
~% 
123948-87-8 |
| Literature: WO2008/127606 A1, ; Page/Page column 5-7 ; |
|
~66% 
123948-87-8 |
| Literature: Puri; Handa; Dhar; Suri; Qazi Synthetic Communications, 2004 , vol. 34, # 15 p. 2857 - 2862 |
|
~63% 
123948-87-8 |
| Literature: Gao, Mingzhang; Miller, Kathy D.; Sledge, George W.; Zheng, Qi-Huang Bioorganic and Medicinal Chemistry Letters, 2005 , vol. 15, # 17 p. 3865 - 3869 |
|
~% 
123948-87-8 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 15, # 17 p. 3865 - 3869 |
| Precursor 6 | |
|---|---|
| DownStream 1 | |
| HS Code | 2942000000 |
|---|