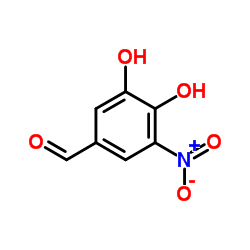
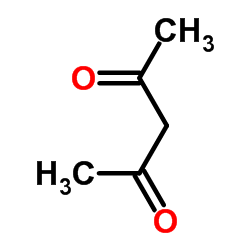
116313-94-1
| Name | 3-[(3,4-dihydroxy-5-nitrophenyl)methylidene]pentane-2,4-dione |
|---|---|
| Synonyms |
3-(3,4-dihydroxy-5-nitrobenzylidene)-2,4-pentanedione
2,4-Pentanedione,3-((3,4-dihydroxy-5-nitrophenyl)methylene) Nitecapone 3-(3,4-dihydroxy-5-nitrophenyl)methylene-2,4-pentanedione Nitecapona [INN-Spanish] Nitecapone [INN] OR 462 Nitecaponum [INN-Latin] |
| Description | Nitecapone (OR-462) is an orally active and short-acting catechol-O-methyltransferase (COMT) inhibitor with gastroprotective and antioxidant properties. Nitecapone (OR-462) scavenges reactive oxygen and nitric radicals and prevents lipid peroxidation[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Nitecapone (1-100 μM) reducesd GSH (reduced glutathione) depletion induced by ROO-by 11-38% and oxidation to oxidized glutathione (GSSG) by 32-45%[1]. |
| In Vivo | Nitecapone (30 mg/kg, ip daily for 13 days) reduces development and symptoms of neuropathic pain after spinal nerve ligation in rats[3]. Animal Model: Eighty-six male Wistar rats, weighing 140-350 g[3]. Dosage: 30 mg/kg (3.3 mL/kg). Administration: IP, once daily for 13 days. Result: Selectively and specifically inhibits COMT in the peripheral tissues, and to some extent in the CNS for ca. 3 h. Increased the thresholds for the mechanical stimuli and thus reduced mechanical allodynia. Reduced the number of positive reactions of the ipsilateral paws when compared with the baselines in the nitecapone-pretreated rats. |
| References |
| Density | 1.451g/cm3 |
|---|---|
| Boiling Point | 495.3ºC at 760 mmHg |
| Molecular Formula | C12H11NO6 |
| Molecular Weight | 265.21900 |
| Flash Point | 211.6ºC |
| Exact Mass | 265.05900 |
| PSA | 120.42000 |
| LogP | 2.09060 |
| Vapour Pressure | 1.95E-10mmHg at 25°C |
| Index of Refraction | 1.645 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| RIDADR | UN 2811 6.1 / PGIII |
|
~45% 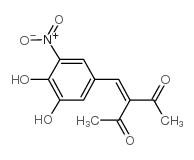
116313-94-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 32, # 4 p. 841 - 846 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |