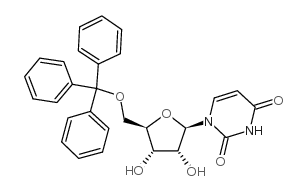
4710-75-2
| Name | 1-[3-hydroxy-4-trityloxy-5-(trityloxymethyl)oxolan-2-yl]pyrimidine-2,4-dione |
|---|---|
| Synonyms |
3',5'-O-ditrityluridine
3',5'-Bis-triphenylmethyl-pyrimidin-ribosid 3',5'-ditrityluridine 3',5'-Di-O-Trityl-uridin O3',O5'-ditrityl-uridine 3',5'-di-O-trityluridine |
| Description | 3′,5′-Bis-O-(triphenylmethyl)uridine is a uridine analog. Uridine has potential antiepileptic effects, and its analogs can be used to study anticonvulsant and anxiolytic activities, as well as to develop new antihypertensive agents[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.33g/cm3 |
|---|---|
| Molecular Formula | C47H40N2O6 |
| Molecular Weight | 728.83 |
| Exact Mass | 728.28900 |
| PSA | 102.78000 |
| LogP | 7.18090 |
| Index of Refraction | 1.705 |
|
~7% 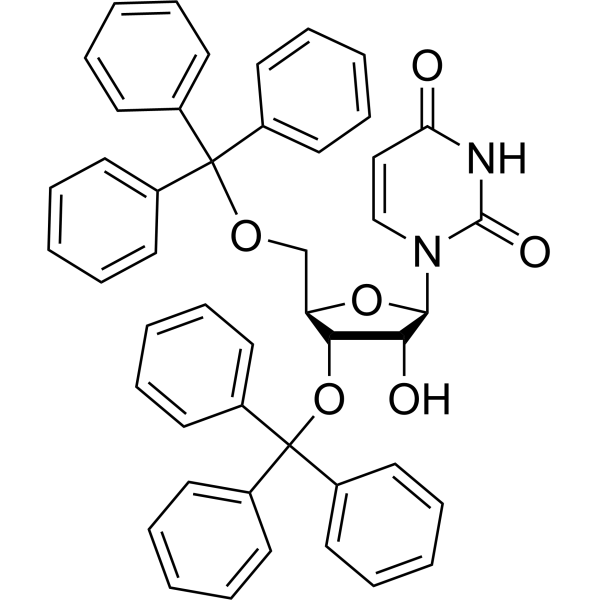
4710-75-2
Detail
|
| Literature: Chatelain, Gregory; Debing, Yannick; De Burghgraeve, Tine; Zmurko, Joanna; Saudi, Milind; Rozenski, Jef; Neyts, Johan; Van Aerschot, Arthur European Journal of Medicinal Chemistry, 2013 , vol. 65, p. 249 - 255 |
|
~% 
4710-75-2 |
| Literature: Chatelain, Gregory; Debing, Yannick; De Burghgraeve, Tine; Zmurko, Joanna; Saudi, Milind; Rozenski, Jef; Neyts, Johan; Van Aerschot, Arthur European Journal of Medicinal Chemistry, 2013 , vol. 65, p. 249 - 255 |
|
~22%
Detail
|
| Literature: Rhie; Pfleiderer Nucleosides and Nucleotides, 1994 , vol. 13, # 6-7 p. 1425 - 1452 |
|
~34% 
4710-75-2 |
| Literature: Rhie; Pfleiderer Nucleosides and Nucleotides, 1994 , vol. 13, # 6-7 p. 1425 - 1452 |
|
~18% 
4710-75-2 |
| Literature: Auguste, Sandra P.; Young, Douglas W. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1995 , # 4 p. 395 - 404 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |