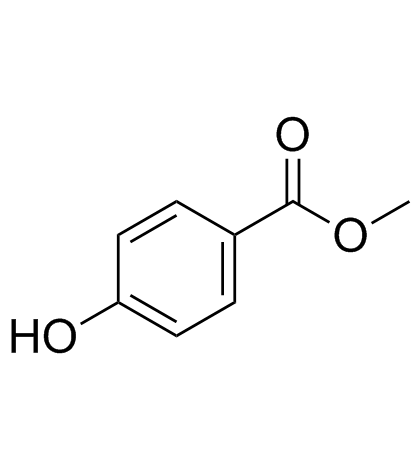
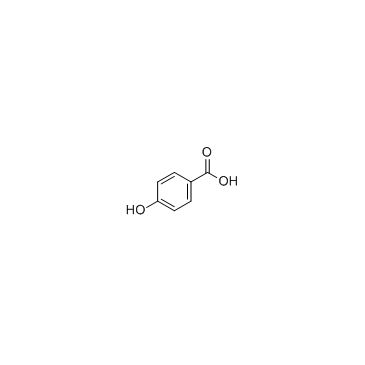
965-52-6
| Name | 4-hydroxy-N-[(E)-(5-nitrofuran-2-yl)methylideneamino]benzamide |
|---|---|
| Synonyms |
Benzoic acid, 4-hydroxy-, 2-[(1E)-(5-nitro-2-furanyl)methylene]hydrazide
Nifuroxazide Ercefuryl Diarlidan Dicoferin EINECS 213-522-0 Nifuroxazid Nifuroxazidum 4-Hydroxy-N'-[(E)-(5-nitro-2-furyl)methylene]benzohydrazide Nifuroxazida p-hydroxy-N'-(5-nitrofurfurylidene)benzhydrazide MFCD00079482 Nifuroxazidum [INN-Latin] Ercefurol |
| Description | Nifuroxazide is an effective inhibitor of STAT3, also exerts potent anti-tumor and anti-metastasis activity. |
|---|---|
| Related Catalog | |
| Target |
STAT3 |
| In Vitro | When U266 cells are incubated with Nifuroxazide, a significant dose-dependent decrease in STAT3 tyrosine phosphorylation is observed. This inhibition of STAT3 tyrosine phosphorylation is rapid, occurring as early as 1 h after treatment, and is sustained for at least 24 h. Treatment of U266 or INA6 cells with Nifuroxazide for 48 hours result in a dose-dependent loss of cell viability with an EC50 of approximately 4.5 μM in both cell types. Notably, the MM cells lacking constitutive STAT3 activation show little toxicity to Nifuroxazide[1]. |
| In Vivo | Compared with the vehicle group, treatment with Nifuroxazide could inhibit tumor growth and tumor weight in a dose-dependent manner, with the inhibition rate of tumor volumes being 43.0% and 62.1% at 25 mg/kg and 50 mg/kg, respectively. It is also shown that Nifuroxazide significantly inhibits the proliferation of nuclear Ki-67-positive cells and induces apoptosis cells of cleaved caspase-3-positive cells. Besides, it is found that treatment with Nifuroxazide could inhibit the expression of MMP-2, MMP-9 and p-Stat3 in A375 tumor tissues. What’s more, Nifuroxazide inhibits the infiltration of MDSCs into the lung, which might be associated with suppression of distant colonization of tumor cells in B16-F10 melanoma metastasis model[2]. |
| Animal Admin | Mice[2] Mice engrafted subcutaneously with 1×107 A375 cells are randomly divided into groups when tumor volume is around 100 mm3 and are administrated intraperitoneally injected with Nifuroxazide 25 mg/kg, 50 mg/kg or vehicle once daily. The tumor size and body weight are measured every 3 days. C57Bl/6J mice are engrafted by injecting intravenously via the tail vein with 2×105 B16-F10 cells to produce experimental lung metastasis. They are randomly assigned to groups on day 6 and are intraperitoneally injected with Nifuroxazide 50 mg/kg or vehicle once daily. Black dots on lung surface are counted and confirmed as melanoma metastases[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Melting Point | 281-283°C |
| Molecular Formula | C12H9N3O5 |
| Molecular Weight | 275.217 |
| Exact Mass | 275.054230 |
| PSA | 120.65000 |
| LogP | 0.59 |
| Index of Refraction | 1.653 |
| Storage condition | Refrigerator, Under Inert Atmosphere |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DH2528300 |
| HS Code | 2928000090 |
|
~83% 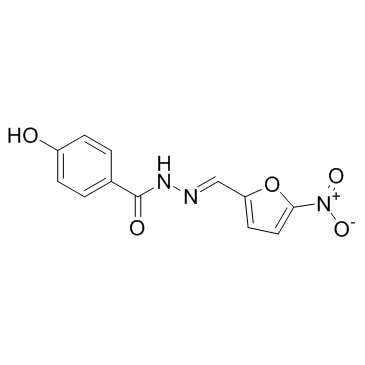
965-52-6 |
| Literature: Tavares; Penna; Amaral Bollettino Chimico Farmaceutico, 1997 , vol. 136, # 3 p. 244 - 249 |
|
~% 
965-52-6 |
| Literature: Bollettino Chimico Farmaceutico, , vol. 136, # 3 p. 244 - 249 |
|
~% 
965-52-6 |
| Literature: Bollettino Chimico Farmaceutico, , vol. 136, # 3 p. 244 - 249 |
| HS Code | 2932190090 |
|---|---|
| Summary | 2932190090 other compounds containing an unfused furan ring (whether or not hydrogenated) in the structure VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |