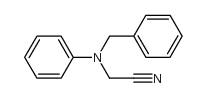
91-75-8
| Name | antazoline |
|---|---|
| Synonyms |
Phenazoline
Antistin Histostab Fenazolina Phenazolin Histazine N-benzyl-N-(4,5-dihydro-1H-imidazol-2-ylmethyl)phenylamine EINECS 202-094-0 Antastan Antistine Antazoline Imidamine Antihistal N-Benzyl-N-(4,5-dihydro-1H-imidazol-2-ylmethyl)aniline N-Benzyl-N-(4,5-dihydro-1H-imidazol-2-ylmethyl)-anilin MFCD00047013 Antasten benzyl-(4,5-dihydro-1H-imidazol-2-ylmethyl)-phenyl-amine N-benzyl-N-(4,5-dihydro-1H-imidazol-2-ylmethyl)-aniline N-(4,5-dihydro-1H-imidazol-2-ylmethyl)-N-(phenylmethyl)aniline |
| Description | Antazoline is an H1 receptor antagonist that affects the activity of the central nervous system, has a potent antiarrhythmic effect[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
H1 receptor |
| In Vitro | Antazoline shows good inhibitory effect on HBV DNA in the supernatant of HepAD38 and Huh7 cells with the value of EC50 is 2.910 μmol/L and 2.349 μmol/L, respectively[2]. Cell Cytotoxicity Assay[2] Cell Line: HepAD38 cells Concentration: 10μmol/L Incubation Time: 5 days Result: Exhibited no significant cytotoxicity at a concentration of 10μmol/L and had a dose-dependent inhibition of HBV DNA in the supernatant. RT-PCR[2] Cell Line: Huh7 cells Concentration: 30 μmol/L, 10 μmol/L, 3.33 μmol/L, 1.1 μmol/L, 0.370 μmol/L, and 0.123 μmol/L Incubation Time: 4 days Result: Had a significant inhibitory effect on HBV DNA in supernatants in a dose-dependent manner. |
| In Vivo | Antazoline (IP; 0.01 ml/g; 30min) as H1 receptor antagonists diminishes the anticonvulsant activity of carbamazepine and diphenylhydantoin[3]. Animal Model: Swiss mice[3] Dosage: 0.01 mL/g Administration: Antazoline (IP; 0.01 ml/g; 30min) Result: Showed some proconvulsive activity. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 475.5±38.0 °C at 760 mmHg |
| Melting Point | 159ºC |
| Molecular Formula | C17H19N3 |
| Molecular Weight | 265.353 |
| Flash Point | 241.4±26.8 °C |
| Exact Mass | 265.157898 |
| PSA | 27.63000 |
| LogP | 4.39 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.608 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| Safety Phrases | S22-S24/25 |
|
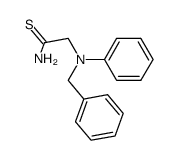
~78% 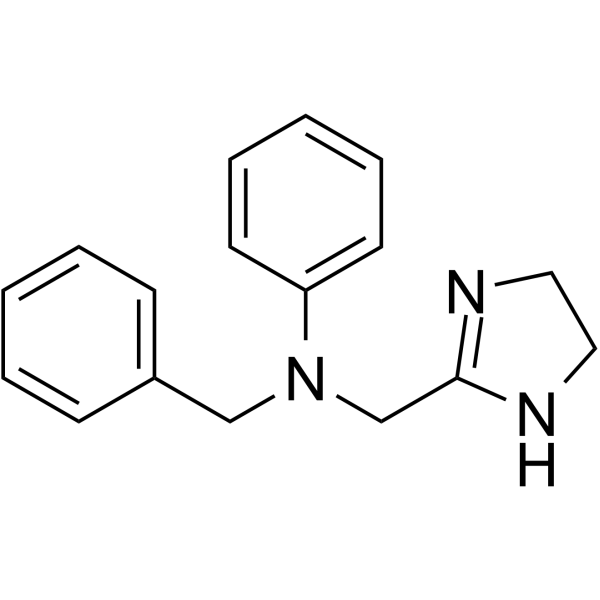
91-75-8 |
| Literature: Dash; Kudav; Parihar Journal of Heterocyclic Chemistry, 2006 , vol. 43, # 2 p. 401 - 404 |
|
~% 
91-75-8 |
| Literature: Helvetica Chimica Acta, , vol. 33, p. 1386,1403 |
|
~% 
91-75-8 |
| Literature: DE847746 , ; DRP/DRBP Org.Chem. US2505248 ; |
|
~% 
91-75-8 |
| Literature: DE847746 , ; DRP/DRBP Org.Chem. US2505248 ; |
|
~% 
91-75-8 |
| Literature: US2505133 , ; |
| Precursor 7 | |
|---|---|
| DownStream 0 | |