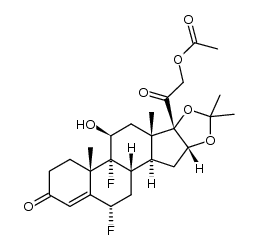
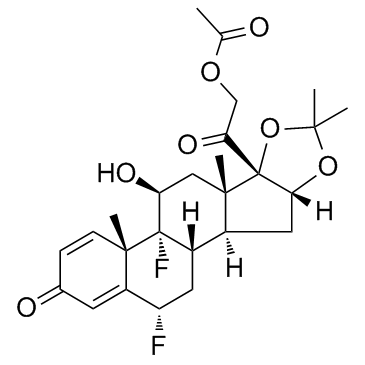
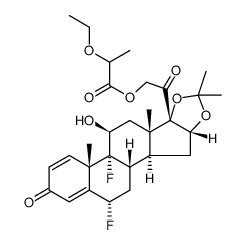
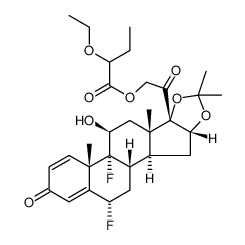
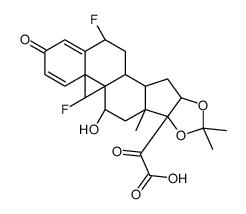
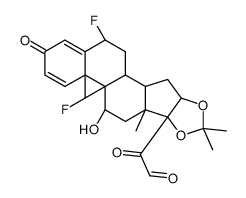
67-73-2
| Name | fluocinolone acetonide |
|---|---|
| Synonyms |
(4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS,12S)-4b,12-difluoro-5-hydroxy-6b-(hydroxyacétyl)-4a,6a,8,8-tétraméthyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodécahydro-2H-naphto[2',1':4,5]indéno[1,2-d][1,3]dioxol-2-one
(4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS,12S)-4b,12-difluoro-5-hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one EINECS 200-668-5 (4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS,12S)-4b,12-difluoro-5-hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2 6a,9a-Difluoro-16a-hydroxyprednisolone 16,17-acetonide MFCD00010525 (6a,11b,16a)-6,9-Difluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione 6a,9a-Difluoro-11b,16a,17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone 6a,9a-Difluoro-16a,17a-isopropylidenedioxy-1,4-pregnadiene-3,20-dione 6α,9α-Difluoro-11β,16α,17α,21-tetrahydroxy-1,4-pregnadiene-3,20-dione Fluocinolone Acetonide (4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS,12S)-4b,12-Difluoro-6b-glycoloyl-5-hydroxy-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one (4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS,12S)-4b,12-Difluor-5-hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-on Fluocinolone (Acetonide) |
| Description | Fluocinolone Acetonide is a glucocorticoid derivative used topically in the treatment of various skin disorders.Target: Glucocorticoid ReceptorFluocinolone acetonide is a corticosteroid primarily used in dermatology to reduce skin inflammation and relieve itching. It is a synthetic hydrocortisone derivative. The fluorine substitution at position 9 in the steroid nucleus greatly enhances its activity. A typical dosage strength used in dermatology is 0.01-0.025%. One such cream is sold under the brand name Flucort-N and includes the antibiotic neomycin. The Glucocorticoid Receptor(GR) binding affinity (IC50) for Fluocinolone Acetonide(FA) was 2.0 nM, respectively. The values is similar to the GR transactivation EC50 of 0.7 nM for FA, respectively [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 578.5±50.0 °C at 760 mmHg |
| Melting Point | 267-269 °C(lit.) |
| Molecular Formula | C24H30F2O6 |
| Molecular Weight | 452.488 |
| Flash Point | 303.7±30.1 °C |
| Exact Mass | 452.201050 |
| PSA | 93.06000 |
| LogP | 2.24 |
| Vapour Pressure | 0.0±3.6 mmHg at 25°C |
| Index of Refraction | 1.577 |
| Storage condition | Refrigerator |
|
Section 1. Chemical Product and Company Identification Fluocinolone acetonide Common Name/ Trade Name Fluocinolone acetonide Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at
least 15 minutes. Get medical attention. Skin ContactIn case of contact, immediately flush skin with plenty of water. Cover the irritated skin with an emollient. Remove contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention. Serious Skin ContactWash with a disinfectant soap and cover the contaminated skin with an anti-bacterial cream. Seek medical attention. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationNot available. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Loosen tight clothing such as a collar, tie, belt or waistband. Get medical attention if symptoms appear. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. Products of CombustionThese products are carbon oxides (CO, CO2), halogenated compounds. Fire Hazards in Presence of Slightly flammable to flammable in presence of heat. Various SubstancesNon-flammable in presence of shocks. Explosion Hazards in Presence Slightly explosive in presence of open flames and sparks. of Various SubstancesNon-explosive in presence of shocks. Fire Fighting MediaSMALL FIRE: Use DRY chemical powder. and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. Special Remarks onAs with most organic solids, fire is possible at elevated temperatures When heated to decomposition it emits toxic Fire Hazardsfumes of Carbon Monoxide, Carbon Dioxide, and Hydrogen Fluoride Special Remarks on Explosion Fine dust dispersed in air in sufficient concentrations, and in the presences of an ignition source is a potential dust Hazardsexplosion hazard. Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Fluocinolone acetonide Section 7. Handling and Storage PrecautionsKeep locked up.. Keep away from heat. Keep away from sources of ignition. Do not ingest. Do not breathe dust. Wear suitable protective clothing. In case of insufficient ventilation, wear suitable respiratory equipment. If ingested, seek medical advice immediately and show the container or the label. Avoid contact with skin and eyes. Keep away from incompatibles such as oxidizing agents. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSplash goggles. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Crystalline solid. Crystalline powder.)OdorOdorless. Not available. Taste Molecular Weight452.49 g/mole White. Color Not applicable. pH (1% soln/water) Boiling PointNot available. Melting Point266°C (510.8°F) Critical TemperatureNot available. Specific GravityNot available. Not applicable. Vapor Pressure Vapor DensityNot available. Not available. Volatility Odor ThresholdNot available. Water/Oil Dist. Coeff.Not available. Ionicity (in Water)Not available. Dispersion PropertiesSee solubility in water, methanol, acetone. SolubilitySoluble in methanol, acetone. Insoluble in cold water, hot water. Soluble in alcohol. Slightly soluble in Chloroform. Fluocinolone acetonide Section 10. Stability and Reactivity Data The product is stable. Stability Instability TemperatureNot available. Excess heat, dust generation, incompatible materials Conditions of Instability Reactive with oxidizing agents. Incompatibility with various substances CorrosivityNon-corrosive in presence of glass. Special Remarks onNot available. Reactivity Special Remarks onNot available. Corrosivity PolymerizationWill not occur. Section 11. Toxicological Information Routes of EntryAbsorbed through skin. Inhalation. Ingestion. Toxicity to AnimalsAcute oral toxicity (LD50): >4000 mg/kg [Mouse]. Chronic Effects on Humans DEVELOPMENTAL TOXICITY: Classified Reproductive system/toxin/female [POSSIBLE]. Other Toxic Effects onHazardous in case of skin contact (irritant), of ingestion, of inhalation. HumansSlightly hazardous in case of skin contact (permeator). Special Remarks onNot available. Toxicity to Animals Special Remarks onMay cause adverse reproductive effects. Chronic Effects on Humans May affect genetic material (mutagenic) Special Remarks on otherPotential Health Effects: Toxic Effects on HumansSkin: May cause skin irritation with itching and burning feeling, skin dryness, folliculitis, acneifom erruptions, perioral dermatitis, allergic contact dermatitis, hypertrichosis. It may be absorbed by the skin and cause systemic effects. Eyes: Causes eye irritation. Inhalation: Causes upper respiratory tract and mucous membrane irritation. Ingestion: May cause gastrointestinal tract irritation. Prolonged or repeated ingestion may affect metabolism Section 12. Ecological Information EcotoxicityNot available. BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. Toxicity of the ProductsThe products of degradation are more toxic than the product itself. of Biodegradation Special Remarks on theNot available. Products of Biodegradation Fluocinolone acetonide Section 13. Disposal Considerations Waste DisposalWaste must be disposed of in accordance with federal, state and local environmental control regulations. Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). Not applicable. Identification Not applicable. Special Provisions for Transport DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms No products were found. Federal and State Regulations California Proposition 65 Warnings Other RegulationsEINECS: This product is on the European Inventory of Existing Commercial Chemical Substances. WHMIS (Canada) Not controlled under WHMIS (Canada). Other Classifications DSCL (EEC)R36/38- Irritating to eyes and skin.S2- Keep out of the reach of children. R40- Possible risks of irreversibleS36/37- Wear suitable protective clothing and effects.gloves. R62- Possible risk of impaired fertility.S46- If swallowed, seek medical advice immediately and show this container or label. Health Hazard HMIS (U.S.A.)2 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 2 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) Fluocinolone acetonide ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22;R36/37/38;R40 |
| Safety Phrases | S26-S36-S22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TU3830000 |
| HS Code | 2937210000 |
|
~% 
67-73-2 |
| Literature: Journal of Labelled Compounds and Radiopharmaceuticals, , vol. 53, # 5-6 p. 260 - 265 |
|
~% 
67-73-2 |
| Literature: Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy, , vol. 75, # 2 p. 930 - 935 |
|
~% 
67-73-2 |
| Literature: Journal of Labelled Compounds and Radiopharmaceuticals, , vol. 52, # 4 p. 103 - 109 |
|
~% 
67-73-2 |
| Literature: Molecules, , vol. 16, # 3 p. 2658 - 2671 |
|
~% 
67-73-2 |
| Literature: Molecules, , vol. 16, # 3 p. 2658 - 2671 |
|
~% 
67-73-2 |
| Literature: Molecules, , vol. 16, # 3 p. 2658 - 2671 |
|
~% 
67-73-2 |
| Literature: Molecules, , vol. 16, # 3 p. 2658 - 2671 |
|
~% 
67-73-2 |
| Literature: Molecules, , vol. 16, # 3 p. 2658 - 2671 |
| Precursor 5 | |
|---|---|
| DownStream 3 | |
| HS Code | 2937210000 |
|---|