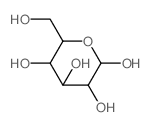
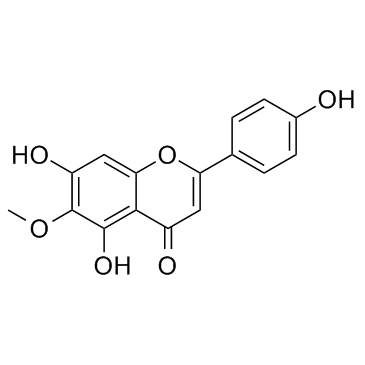
17680-84-1
| Name | 5-hydroxy-2-(4-hydroxyphenyl)-6-methoxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one |
|---|---|
| Synonyms |
hispidulin-7-glucoside
4H-1-Benzopyran-4-one, 7-(β-D-glucopyranosyloxy)-5-hydroxy-2-(4-hydroxyphenyl)-6-methoxy- 5-Hydroxy-2-(4-hydroxyphenyl)-6-methoxy-4-oxo-4H-chromen-7-yl β-D-glucopyranoside HISPIDULOSIDE Hispidulin 7-O-glucoside Homoplantaginin |
| Description | Homoplantaginin is a flavonoid from a traditional Chinese medicine Salvia plebeia with antiinflammatory and antioxidant properties. Homoplantaginin could inhibit TNF-α and IL-6 mRNA expression, IKKβ and NF-κB phosphorylation. |
|---|---|
| Related Catalog | |
| Target |
TNF-α p65 |
| In Vitro | Homoplantaginin shows IC50 of reduction level of DPPH radical at 0.35 μg/mL. In human hepatocyte HL-7702 cells exposed to H2O2, the addition of 0.1-100μg/mL of homoplantaginin significantly reduces lactate dehydrogenase leakage, and increases glutathione, glutathione peroxidase and superoxide dismutase in supernatant[1]. Homoplantaginin (0.1, 1, 10 μM) dose-dependently reduces expression of toll-like receptor-4 evoked by palmitic acid (100 μM). Homoplantaginin tightly controlls palmitic acid-induced reactive oxygen species to prevent nucleotide-binding domain-like receptor 3 (NLRP3) inflammasome activation by suppressing reactive oxygen species-sensitive thioredoxin-interacting protein, NLRP3, and caspase-1[2]. Pre-treatment of homoplantaginin on human umbilical vein endothelial cells significantly inhibits palmitic acid induced TNF-α and IL-6 mRNA expression, and IKKβ and NF-κB p65 phosphorylation. Homoplantaginin significantly modulates the Ser/Thr phosphorylation of IRS-1, improves phosphorylation of Akt and endothelial nitric oxide synthase, and increases NO production in the presence of insulin[3]. |
| In Vivo | Homoplantaginin(25-100mg/kg) significantly reducesthe increase in serum alanine aminotranseferase and aspartate aminotransferase, decreases the levels of TNF-α and IL-1. The same treatment also reduces the content of thiobarbituric acid-reactive substances, elevates the levels of GSH, GSH-Px and SOD in hepatic homogenate[1]. Homoplantaginin is rapidly absorbed (Tmax=16.00±8.94min), reaching a mean Cmax between 0.77 and 1.27nmol/mL. The absolute oral bioavailability is calculated to be only 0.75%. |
| Cell Assay | The viability of cultured cells is determined using the MTT assay. Human umbilical vein endothelial cells are exposed to various concentrations of homoplantaginin (0.1, 1, 3, 10, 30, 100 μM) for 48 h. Subsequently, 20 μL of MTT (5 mg/mL) is added to each well for an additional 4 h at 37°C. The supernatant is removed, and DMSO is added to dissolvethe formazan crystals. The optical absorbance is measured at 540 nm[3]. |
| Animal Admin | Rats: Homoplantaginin is dissolved in a solution consistingof DMSO, PEG 400, ethanol and normal saline(2:2:3:3, v/v/v/v) at a concentration of 10 mg/mL. The rats are randomly divided into three groups to receive oral administration(150 mg/kg), tail vein injection (15 mg/kg) and peritoneal injectionv (15 mg/kg). Blood samples (approximately 0.5 mL) are collected from the retro-orbital plexus into heparinized microfugetubes at 5, 10, 20, 30, 45, 60, 90, 120, and 180 min after administration. The plasma samples, separated by centrifuging the blood samples at 10,000 rpm[4]. Mice: Homoplantaginin is dissolved in 5% amylum. Homoplantaginin is administered orally by gastric intubation during the experimental period at doses of 25, 50, 100 mg/kg/d, respectively. Eight hours after injection of LPS, the mice are anesthetized with ether and blood samples are collected by exsanguination from the inferior vein. The liver is removed and fixed in formalin for histological analysis[1]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 798.1±60.0 °C at 760 mmHg |
| Melting Point | 256-258℃ |
| Molecular Formula | C22H22O11 |
| Molecular Weight | 462.404 |
| Flash Point | 279.7±26.4 °C |
| Exact Mass | 462.116211 |
| PSA | 179.28000 |
| LogP | -0.97 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.695 |
| Storage condition | -20°C |