91832-40-5
| Name | cefdinir |
|---|---|
| Synonyms |
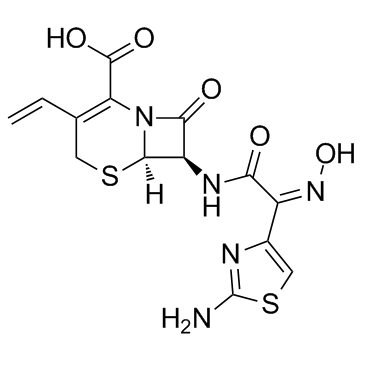
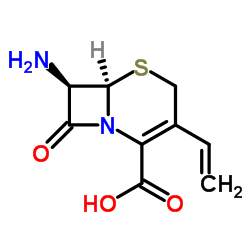
(6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(hydroxyimino)acetyl]amino}-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Omnicef 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2Z)-2-(2-amino-4-thiazolyl)-2-(hydroxyimino)-1-oxoethyl]amino]-3-ethenyl-8-oxo-, (6R,7R)- Cefzon (6R,7R)-7-{[(2E)-2-(2-Amino-1,3-thiazol-4-yl)-2-(hydroxyimino)acetyl]amino}-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Novacef Cefdinir FK-482 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2E)-2-(2-amino-4-thiazolyl)-2-(hydroxyimino)-1-oxoethyl]amino]-3-ethenyl-8-oxo-, (6R,7R)- cefdinyl CFDN CI 983 MFCD00865030 [6R-[6a,7b(Z)]]-7-[[(2-Amino-4-thiazolyl)(hydroxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (6R,7R)-7-{[(2E)-2-(2-amino-1,3-thiazol-4-yl)-2-(hydroxyimino)acetyl]amino}-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (6R,7R)-7-{[(2Z)-2-(2-Amino-1,3-thiazol-4-yl)-2-(hydroxyimino)acetyl]amino}-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Omicef Omnice EINECS 643-088-1 |
| Description | Cefdinir (Omnicef) is a semi-synthetic, broad-spectrum antibiotic, which is proved to be effective for common bacterial infections of the ear, sinus, throat, and skin.Target: AntibacterialCefdinir is a third generation oral cephalosporin antibiotic. Cefdinir (Omnicef) is a semi-synthetic, broad-spectrum antibiotic in the third generation of the cephalosporin class, which is proved to be effective for common bacterial infections of the ear, sinus, throat, and skin. It can be used to treat infections caused by several Gram-negative and Gram-positive bacteria. It is available in US as Omnicef by Abbott Laboratories and in India as Cednir by Abbott, Kefnir by Glenmark and Cefdiel by Ranbaxy. As of 2008, cefdinir was the highest-selling cephalosporin antibiotic in the United States, with more than US$585 million in retail sales of its generic versions alone.Cefdinir, a new oral 2-amino-5-thiazolyl cephalosporin, inhibited the luminol-amplified chemiluminescence (LACL) response of human neutrophils stimulated by PMA but not opsonized zymosan, in a concentration-dependent but not time-dependent manner. The LACL response to opsonized zymosan in cytochalasin B-treated neutrophils was, however, inhibited by cefdinir. Furthermore, cefdinir inhibited LACL generation in cell-free systems consisting of H2O2, NaI, and either horseradish peroxidase or a myeloperoxidase-containing neutrophil extract. Orthodianisidine oxidation in these two acellular systems was inhibited by cefdinir. |
|---|---|
| Related Catalog | |
| References |
[1]. Soejima R. Cefdinir. Jpn J Antibiot. 1992 Oct;45(10):1239-52. |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Melting Point | >180°C dec. |
| Molecular Formula | C14H13N5O5S2 |
| Molecular Weight | 395.414 |
| Exact Mass | 395.035797 |
| PSA | 211.75000 |
| LogP | -0.63 |
| Index of Refraction | 1.862 |
| Storage condition | Room temp |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | XI0367250 |
| HS Code | 2941905990 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2941905990 |
|---|


![7-(Z)-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-vinyl-3-cephem-4-carboxylic acid ammonium salt structure](https://image.chemsrc.com/caspic/167/696592-20-8.png)
![benzhydryl 7-[2-(-aminothiazaol-4-yl)-2-hydroxyiminoacetamido]-3-vinyl-3-cephem-4-carboxylate hydrobromide (syn isomer) structure](https://image.chemsrc.com/caspic/289/1056963-31-5.png)
![N,N'-dicyclohexylethane-1,2-diamine salt of 7β-[2-(2-amino-4-thiazolyl)-2-((Z)-hydroxyimino)acetamido]-3-vinyl-3-cephem-4-carboxylic acid structure](https://image.chemsrc.com/caspic/142/865411-26-3.png)