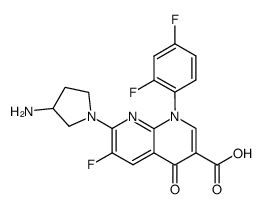
100490-36-6
| Name | tosufloxacin tosilate |
|---|---|
| Synonyms |
Toskisasin
TOSUFLOXACIN TFLX Ozex MFCD01711971 TETRAETHYL AMMONIUM IODIDE TOSUFLOXACIN, ANTIBIOTIC FOR CULTURE MEDIA USE ONLY |
| Description | Tosufloxacin (A-61827) is an orally active fluoroquinolone antibiotic. Tosufloxacin shows a broad spectrum of antibacterial activity against gram-positive and gram-negative bacteria[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Tosufloxacin tosylate hydrate (T-3262) (0.05-3.13 μg/mL; 18 h) shows antibacterial activities against S. aureus, Staphylococcus epidermidis, streptococci, enterococci, Bacteroides fragilis, Clostridium difficile, and Clostridium perfringens[2]. Cell Viability Assay[2] Cell Line: S. aureus, Staphylococcus epidermidis, streptococci, enterococci, Bacteroides fragilis, Clostridium difficile, and Clostridium perfringens Concentration: 0.05-3.13 μg/mL Incubation Time: 18 hours Result: Showed MIC90s (MICs for 90% of the isolates tested) ranging from 0.05 to 1.56 μg/mL for S. aureus, Staphylococcus epidermidis, streptococci, and enterococci. Showed MIC90s of 1.56, 3.13, and 0.20 μg/mL for Bacteroides fragilis, Clostridium difficile, and Clostridium perfringens, respectively. |
| In Vivo | Tosufloxacin tosylate hydrate (T-3262) (oral gavage; 0.16-13.39 mg/kg; once) treatment shows antibacterial activity against S. aureus, E. coli, and P. aeruginosa in vivo[2]. Animal Model: Male Slc:ICR mice infected with S. aureus[2] Dosage: 1.27-2.15 mg/kg Administration: Oral gavage; 1.27-2.15 mg/kg; once Result: Showed 50% effective dose (ED50) of 1.62 mg/kg (body weight) at 7 days after infection. Showed MIC value of 0.0125 μg/mL. Animal Model: Male Slc:ICR mice infected with E. coli[2] Dosage: 0.16-0.30 mg/kg Administration: Oral gavage; 0.16-0.30 mg/kg; once Result: Showed 50% effective dose (ED50) of 0.22 mg/kg (body weight) at 7 days after infection. Showed MIC value of 0.0125 μg/mL. Animal Model: Male Slc:ICR mice infected with P. aeruginosa[2] Dosage: 7.66-13.39 mg/kg Administration: Oral gavage; 7.66-13.39 mg/kg; once Result: Showed 50% effective dose (ED50) of 10.13 mg/kg (body weight) at 7 days after infection. Showed MIC value of 0.78 μg/mL. |
| Density | 1.558g/cm3 |
|---|---|
| Boiling Point | 614.4ºC at 760 mmHg |
| Molecular Formula | C19H15F3N4O3 |
| Molecular Weight | 404.34300 |
| Flash Point | 325.4ºC |
| Exact Mass | 404.11000 |
| PSA | 101.45000 |
| LogP | 2.80390 |
| Vapour Pressure | 5.96E-16mmHg at 25°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26-S37/39 |
| WGK Germany | 3 |