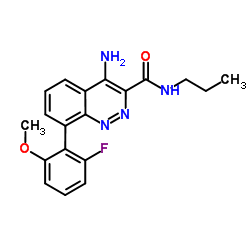
942437-37-8
| Name | AZD-7325 |
|---|---|
| Synonyms |
KNM216XOUH
4-Amino-8-(2-fluoro-6-methoxyphenyl)-N-propyl-3-cinnolinecarboxamide AZD-7325 |
| Description | AZD7325 is a potent and orally active partial selective PAM of GABAAα2 and Aα3 receptor (Ki=0.3 and 1.3 nM, respectively), and has less antagonistic efficacy at the Aα1 and Aα5 receptor subtypes[1][4]. AZD7325 is a moderate CYP1A2 and a potent CYP3A4 inducer in vitro[2]. AZD7325 has the potential for the investigation of anxiety and dravet syndrome[3]. PAM: positive allosteric modulator. |
|---|---|
| Related Catalog | |
| Target |
CYP1A2 CYP3A4 |
| In Vitro | AZD7325 is a high affinity and selective modulator of the GABAA receptor system, exhibits high binding affinity at GABAAα1, α2 and α3 (Ki=0.5, 0.3, and 1.3 nM, respectively), and low at GABAAα5 (Ki=230 nM)[4]. AZD7325 (0-10 µM; 3 consecutive days; once daily) causes a maximal CYP1A2 mRNA expression of 3.2-fold, 2.1-fold, and 2.5-fold in human hepatocytes from donor HH210, HH215, and HH216, respectively[2]. AZD7325 (0-10 µM; 3 consecutive days; once daily) causes CYP1A2 and CYP3A4 protein expression in human hepatocytes from donor HH210[2]. RT-PCR[2] Cell Line: Primary human hepatocytes from one female (HH210) and two male (HH215, HH216) donors Concentration: 0.01, 0.1, 1, 10 µM Incubation Time: 3 consecutive days Result: Led to increase of CYP1A2 mRNA expression Western Blot Analysis[2] Cell Line: Primary human hepatocytes from donors Concentration: 0.01, 0.1, 1, 10 µM Incubation Time: 3 consecutive days Result: Increased CYP1A2 and CYP3A4 protein level. |
| In Vivo | AZD7325 (oral administration; 10, 17.8 or 31.6 mg/kg; 30 minutes before the induction of hyperthermia) attenuates hyperthermia-induced seizures, shows median thresholds in the treatment groups of 42.8°C for 10 mg/kg, 43.3°C for 17.8 mg/kg, and 43.4°C for 31.6 mg/kg compares to 42.2°C in vehicle group[3]. Animal Model: Male and female P18 - P20 F1.Scn1a+/- mice[3] Dosage: 10, 17.8 or 31.6 mg/kg Administration: Oral administration; 30 minutes before the induction of hyperthermia Result: Attenuated hyperthermia-induced seizures in F1.Scn1a+/- mice with no sedative effect. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Molecular Formula | C19H19FN4O2 |
| Molecular Weight | 354.378 |
| Exact Mass | 354.149200 |
| LogP | 3.83 |
| Index of Refraction | 1.625 |