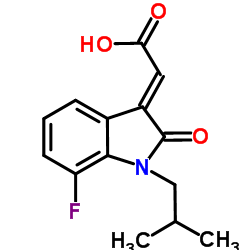
1190217-35-6
| Name | (2E)-(7-Fluoro-1-isobutyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)acetic acid |
|---|---|
| Synonyms |
(2E)-(7-Fluoro-1-isobutyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)acetic acid
ASP 7663 |
| Description | ASP7663 is an orally active and selective TRPA1 agonist. ASP7663 exerts both anti-constipation and anti-abdominal pain actions[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | ASP7663 concentration dependently increases intracellular Ca2+ concentration in human, rat, and mouse TRPA1 expressed in HEK293 cells in a similar manner, with respective EC50 values (95% confidence interval [CI]) of 0.51 (0.40–0.66), 0.54 (0.41–0.72), and 0.50 (0.41–0.63) μmol/L[1]. ASP7663 concentration-dependently stimulates 5-HT release from QGP-1 cells, a lineage of TRPA1-expressing EC cells, with an EC50 value of 72.5 (52.6–99.9) μmol/L[1]. |
| In Vivo | ASP7663 significantly improves the loperamide-induced delay in colonic transit in mice[1]. ASP7663 (orally, 0.3 and 1 mg/kg) significantly shortens the prolonged bead expulsion time caused by loperamide[1]. ASP7663 (orally, 1 and 3 mg/kg) exhibits inhibitory effects on colorectal distension in rat[1]. Animal Model: CRD model (colorectal distension in rat)[1]. Dosage: 1 and 3 mg/kg. Administration: Orally. Result: Significantly reduced the number of abdominal contractions evoked during CRD at pressures of 30, 45, and 60 mmHg. ASP7663 also reduced the number of abdominal contractions by intravenous treatment. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 368.1±42.0 °C at 760 mmHg |
| Molecular Formula | C14H14FNO3 |
| Molecular Weight | 263.264 |
| Flash Point | 176.4±27.9 °C |
| Exact Mass | 263.095764 |
| LogP | 2.82 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.628 |
| RIDADR | NONH for all modes of transport |
|---|