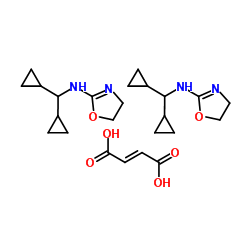
207572-68-7
| Name | Rilmenidine hemifumarate salt |
|---|---|
| Synonyms |
N-(Dicyclopropylmethyl)-4,5-dihydro-1,3-oxazol-2-amine (2E)-but-2-enedioate (2:1)
MFCD00673925 N-(Dicyclopropylmethyl)-4,5-dihydro-1,3-oxazol-2-amine (2E)-2-butenedioate (2:1) 2-Oxazolamine, N-(dicyclopropylmethyl)-4,5-dihydro-, (2E)-2-butenedioate (2:1) MFCD09878261 |
| Description | Rilmenidine hemifumarate, an innovative antihypertensive agent, is an orally active, selective I1 imidazoline receptor agonist. Rilmenidine hemifumarate is an alpha 2-adrenoceptor agonist. Rilmenidine hemifumarate induces autophagy. Rilmenidine hemifumarate acts both centrally by reducing sympathetic overactivity and in the kidney by inhibiting the Na+/H+ antiport. Rilmenidine hemifumarate modulates proliferation and stimulates the proapoptotic protein Bax thus inducing the perturbation of the mitochondrial pathway and apoptosis in human leukemic K562 cells [1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Rilmenidine provides antihypertensive efficacy comparable with that of diuretics, beta-blockers, calcium channel blockers, and angiotensin-converting enzyme (ACE) inhibitors[1]. Rilmenidine (25-100 μM; 24 hours) inhibits K562 cell proliferation[2]. Cell Viability Assay[2] Cell Line: K562 cells Concentration: 25, 50, 100 μM Incubation Time: 24 hours Result: Dose-dependently inhibited K562 colony formation. |
| In Vivo | Rilmenidine-treated N171-82Q mice (i.p.; 4-times a week) displays significant improved forelimb grip strength and all limbs grip strength from 12 to 22 weeks of age[3]. Rilmenidine decreases levels of mutant huntingtin[3]. |
| References |
[1]. Reid JL. Rilmenidine: a clinical overview. Am J Hypertens. 2000;13(6 Pt 2):106S-111S. [3]. Reid JL. Rilmenidine: a clinical overview. Am J Hypertens. 2000;13(6 Pt 2):106S-111S. |
| Molecular Formula | C24H36N4O6 |
|---|---|
| Molecular Weight | 476.566 |
| Exact Mass | 476.263489 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22;S24/25 |
| RIDADR | NONH for all modes of transport |
| RTECS | RP7207400 |