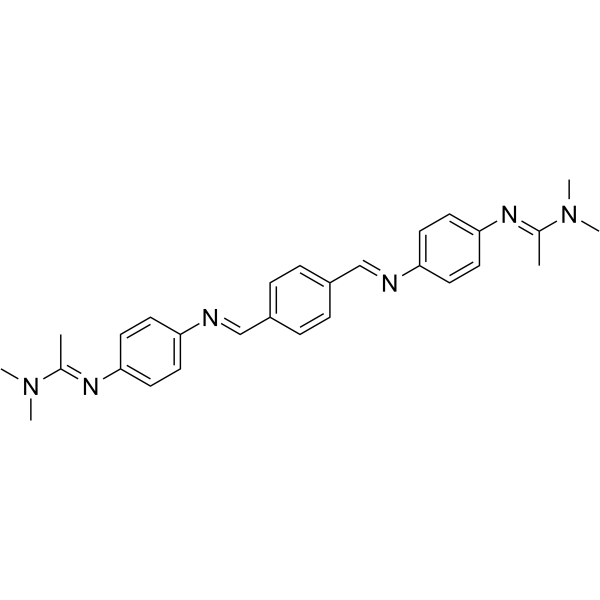
115103-15-6
| Name | N'-[4-[[4-[[4-[1-(dimethylamino)ethylideneamino]phenyl]iminomethyl]phenyl]methylideneamino]phenyl]-N,N-dimethylethanimidamide |
|---|---|
| Synonyms |
Tribendimidine
Tribendimidin |
| Description | Tribendimidine is an orally active, broad-spectrum anthelmintic agent, with particularly high activity against A. lumbricoides and N. americanus. Tribendimidine is also an L-type nicotinic acetylcholine receptor (nAChR) agonist[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Tribendimidine (100 μg/mL, 24 h) shows intoxication of C. elegans[3]. Tribendimidine (0-100 μg/mL, 6 days) shows toxicity with an LC50 value (concentration at which half the animals are dead) of 54.4 μg/mL[3]. Tribendimidine (0-200 μg/mL, 64 h)-induced sterility is resisted by trb mutant hermaphrodites, and Levamisole-resistant mutants are resistant to Tribendimidine. [3]. |
| In Vivo | Tribendimidine (75 and 150 mg/kg; p.o.; once) shows inhibition activity against C. sinensis in rats, and shows inhibition against O. viverrini at 400 mg/kg in hamsters[2]. Animal Model: C. sinensis infected Female Wistar rats[2] Dosage: 75 mg/kg and 150 mg/kg Administration: Oral administration, once Result: A 99.1% worm burden reduction was achieved at 150 mg/kg, and the worm burden reduction was still significant (68.9%) at 75 mg/kg. |
| References |
| Density | 1.04g/cm3 |
|---|---|
| Boiling Point | 618.2ºC at 760mmHg |
| Molecular Formula | C28H32N6 |
| Molecular Weight | 452.59400 |
| Flash Point | 327.7ºC |
| Exact Mass | 452.26900 |
| PSA | 55.92000 |
| LogP | 6.41080 |
| Vapour Pressure | 3.26E-15mmHg at 25°C |
| Index of Refraction | 1.575 |
| Storage condition | 2-8°C |