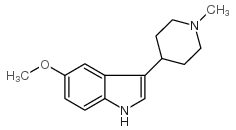
57477-39-1
| Name | 3-(1-methylpiperidin-4-yl)-1H-indol-5-ol |
|---|---|
| Synonyms |
5-hydroxy-3-(1-methylpiperidin-4-yl)indole
UNII-Q2DH1CHI0Y 3-(1-methyl-piperidin-4-yl)-indol-5-ol 3-(1-Methylpiperidin-4-yl)-1H-indol-5-ol BRL-54443 BRL 54443 Tocris-1129 3-(1-Methyl-4-piperidinyl)-1H-indol-5-ol Lopac-B-173 3-(1-methylpiperidin-4-yl)1H-indol-5-ol |
| Description | BRL 54443 is a potent 5-HT1E/1F receptor agonist (pKi values are 8.7 and 8.9 respectively); displays > 30-fold selectivity over other 5-HT and dopamine receptors.IC50 value: 8.7(pKi, 5-HT1E); 8.9 (pKi, 5-HT1F) Target: 5-HT1E/1F receptorin vitro: BRL 54443 is a potent 5-ht1E/1F receptor agonist (pEC50 values are 8.5 and 8.6 respectively). Displays > 30-fold selectivity over other 5-HT and dopamine receptors (pKi values are 8.7. 8.9, 7.2, 6.9, 7.2, 5.9, 7.0, 6.5, < 6, < 6, 6.3 and 6.2 for human 5-HT1E, 1F, 1A, 1B, 1D, 2A, 2B, 2C, 4, 7, D2 and D3 receptors respectively). Induces 5-HT2A receptor-mediated mouse aortic contraction in vitro (pEC50 = 6.52). Active in vivo. In DG membranes, BRL54443, a 5-ht(1E) /5-HT(1F) agonist, selectively stimulated 5-ht(1E) receptors and potently inhibited forskolin-dependent cAMP production (IC50 = 14 nM) [2]. The 5-HT(1E/1F) receptor agonist BRL 54443 also induced contraction (-log EC(50) = 6.52) [1].in vivo: Reduction of flinching was considered as antinociception. Ipsilateral, but not contralateral, peripheral administration of BRL54443 (5-HT(1E/1F); 3-300 microg/paw) significantly reduced formalin-induced flinching in rats [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 431.5±45.0 °C at 760 mmHg |
| Molecular Formula | C14H18N2O |
| Molecular Weight | 230.305 |
| Flash Point | 214.8±28.7 °C |
| Exact Mass | 230.141907 |
| PSA | 39.26000 |
| LogP | 1.16 |
| Appearance | solid | white |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.646 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: 50 mg/mL |
|
~85% 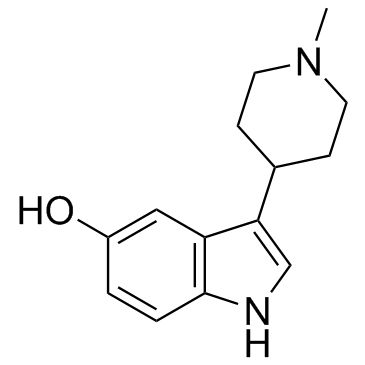
57477-39-1 |
| Literature: Eli Lilly and Company Patent: US6358972 B1, 2002 ; US 6358972 B1 |
|
~% 
57477-39-1 |
| Literature: Medicinal Chemistry Research, , vol. 5, # 9 p. 680 - 689 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |