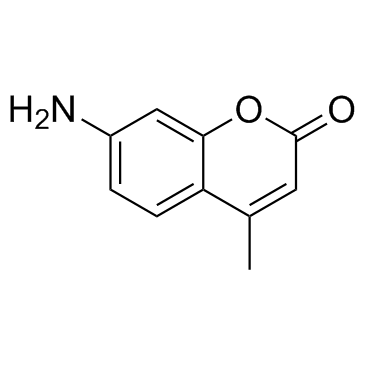
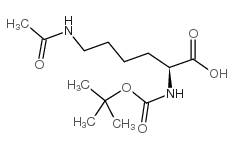
233691-67-3
| Name | tert-butyl N-[(2S)-6-acetamido-1-[(4-methyl-2-oxochromen-7-yl)amino]-1-oxohexan-2-yl]carbamate |
|---|---|
| Synonyms |
(S)-tert-Butyl (6-acetamido-1-((4-methyl-2-oxo-2H-chromen-7-yl)amino)-1-oxohexan-2-yl)carbamate
Histone Deacetylase Substrate,Fluorogenic Nalpha-Boc-Nepsilon-acetyl-L-lysine 7-amido-4-methylcoumarin N-(4-Methyl-7-coumarinyl)-Nalpha-(t-butoxycarbonyl)-Nomega-acetyllysinamide Boc-Ac-Lys-AMC Boc-Lys(Ac)-AMC Boc-L(Ac)-AMC Boc-Lys(Ac)-7-Amino-4-Methylcoumarin tert-Boc (N-acetyl-Lys)-AMC |
| Description | Boc-Lys(Ac)-AMC is a cell-permeable fluorometric HDAC substrate (Ex/Em = 355 nm/460 nm)[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Guidelines (Following is our recommended protocol. This protocol only provides a guideline, and should be modified according to your specific needs). 基于组织培养的 HDAC 检测[2] 1. 将 6 × 104 细胞在 100 mL 培养基中加入 25 μM Boc-Lys(Ac)-AMC 于 96 孔组织培养板中培养过夜。 2. 37°C,5% CO2 下孵育 2-3 小时。 3. 加入含有细胞裂解缓冲液和胰蛋白酶试剂的 HADC 显影液,终止脱乙酰反应。 4. 室温孵育 15 分钟。 5. 在 Ex/Em = 355 nm/460 nm 处,用 SpectraMax M5 微板仪测量 AMC 的荧光信号。 |
| References |
| Molecular Formula | C23H31N3O6 |
|---|---|
| Molecular Weight | 445.50900 |
| Exact Mass | 445.22100 |
| PSA | 126.74000 |
| LogP | 4.09440 |
| Hazard Codes | Xi |
|---|
|
~38% 
233691-67-3 |
| Literature: Hoffmann; Brosch; Loidl; Jung Pharmazie, 2000 , vol. 55, # 8 p. 601 - 606 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |