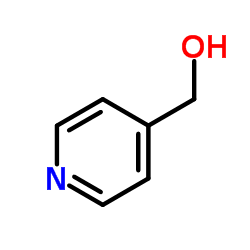
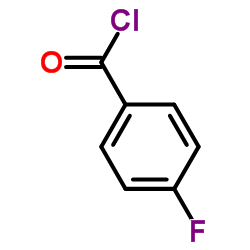
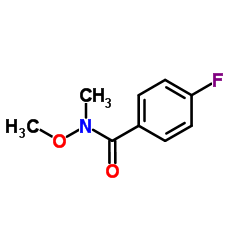
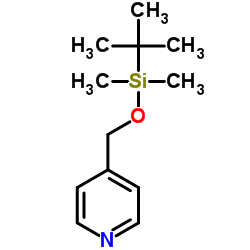
152121-47-6
| Name | 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine |
|---|---|
| Synonyms |
PB 203580
4-(4-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-5-yl)pyridine RWJ 64809 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(pyridin-4-yl)imidazole 4-(4-Fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-5-(4-pyridyl)-1H-imidazole MFCD00922198 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole 4-{4-(4-Fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-1H-imidazol-5-yl}pyridine 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(pyridin-4-yl)-1H-imidazole Pyridine,4-(5-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-4-yl) SB 203580 SB203580 SB-203580 |
| Description | SB 203580 is a widely used p38 MAPK inhibitor with an IC50 of 0.3-0.5 μM. It shows more than 100-fold selectivity over PKB, LCK, and GSK-3β. |
|---|---|
| Related Catalog | |
| Target |
p38 MAP kinase:0.3-0.5 μM (IC50, in IL-2-stimulated T cells) PKB:3-5 μM (IC50, in IL-2-stimulated T cells) Autophagy Mitophagy |
| In Vitro | SB 203580 inhibits IL-2-driven T cell proliferation with an IC50 of 3-5 μM, SB 203580 is able to inhibit the activity of PDK1 in a dose-dependent manner with an IC50 in the 3-10 μM range[1]. SB 203580 at a concentration of 1 μM is sufficient for inhibiting p38 kinase activity in TF-1 cells. SB 203580 at 5 and 10 μM enhances NF-κB-mediated gene transcription independently of phosphorylation on the transactivation domains of the p65 subunit. SB 203580 at 10 μM enhances phosphorylation of ERK1/2 and JNK[1]. |
| In Vivo | All animals challenged with NS (noninfected controls) and treated with either SB203580 or placebo survive. Compared with placebo, pretreatment with the highest dose of SB203580 (100 mg/kg) 1 hour before E. coli increases the hazards ratio of death. With E. coli, compared with placebo, at 48 hours, but not 24 hours, low and high dose SB203580 decrease phosphorylated p38 MAPK and the ratio of phosphorylated to total p38. High dose SB203580 decreases lung neutrophils on histology at 24 hours in a trend approaching significance (p = 0.09) and increases them significantly at 48 hours (p = 0.01) in patterns different over time[3]. SB 203580 is evaluated in several models of cytokine inhibition and inflammatory disease. It is demonstrated clearly to be a potent inhibitor of inflammatory cytokine production in both mice and rats with IC50 values of 15 to 25 mg/kg[4]. |
| Cell Assay | Phosphorylation of p38, JNK1/2, and ERK1/2 is analysed by Western blotting. Briefly, TF-1 cells are cultured for 16 h in RPMI 1640 containing 0.1% FBS and subsequently stimulated for various periods of time with medium or OA (30 ng/mL) or SB 203580(1 μM, 5 μM, 10 μM) plus OA. After harvesting, total cell extracts are prepared by resuspending the cells in 500 μL 1× sample buffer (containing 2% SDS, 10% glycerol, 2% β-mercaptoethanol, 60 mM Tris-HCl (pH 6.8) and bromophenol blue) and lysing the cells by passing them through a 23G1 needle (three times). Cell extracts are directly boiled for 10 min and stored at -20°C. Before loading, samples are again boiled for 5 min and cell extracts are resolved by running 1/10th volume on a SDS/12.5%PAGE gel (acryla-mide:bisacrylamide is 173:1) and transferred to cellulosenitrate membrane. Immunoblotting with the antibodies is performed by standard procedures and detection is performed[2]. |
| Animal Admin | Mice[3] In survival studies, C57BL/6J mice weighing 20 g to 30 g are briefly anesthetized with isoflurane and challenged with 0.05 mL of IT normal saline (NS, noninfected controls) or E. coli (15 × 109 CFU/kg). One hour before NS challenge, mice (n = 24) receive either intraperitoneal SB203580 (100 mg/kg in 0.25 mL) or diluent only (placebo). Infected animals receive SB203580 in doses of 100, 10, 1, or 0.1 mg/kg or placebo 1 hour before IT E. coli (n = 241); SB203580 100 or 0.1 mg/kg or placebo 1 hour after E. coli (n = 121); or SB203580 100 mg/kg or placebo 12 hours after E. coli (n = 72). All animals receive ceftriaxone (100 mg/kg in 0.1 mL,subcutaneously) for 4 days and NS (0.5 mL, subcutaneously) for 1 day beginning 4 hoursafter challenge. Animals were observed every 2 hours for the initial 48 hours, every 4 hours from 48 hours to 72 hours, every 8 hours from 72 hours to 96 hours, and then twice daily until study completion (168 hours)[3]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 615.6±55.0 °C at 760 mmHg |
| Melting Point | 249 - 250ºC |
| Molecular Formula | C21H16FN3OS |
| Molecular Weight | 377.435 |
| Flash Point | 326.1±31.5 °C |
| Exact Mass | 377.099823 |
| PSA | 77.85000 |
| LogP | 4.10 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.715 |
| Storage condition | −20°C |
| Stability | -200C |
| Water Solubility | DMSO: 50 mg/mL |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H318 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22;R41 |
| Safety Phrases | 26-39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2818200000 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |
| HS Code | 2818200000 |
|---|