84294-96-2
| Name | Enoxacin sesquihydrate |
|---|---|
| Synonyms |
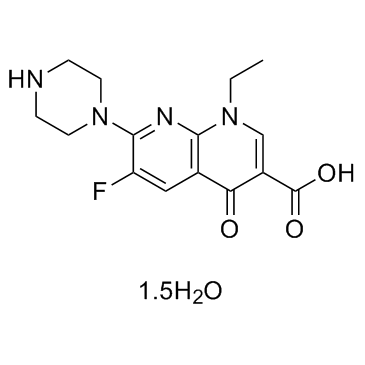
1,8-Naphthyridine-3-carboxylic acid, 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-, hydrate (3:2)
1,8-Naphthyridine-3-carboxylic acid, 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-, hydrate (2:3) 1-Ethyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid hydrate (3:2) Enoxacin times 1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8-naphthyridine-3-carboxylic Acid Enoxacin (hydrate) |
| Description | Enoxacin is a broad-spectrum 6-fluoronaphthyridinone antibacterial agent.Target: antibacterialEnoxacin is a new quinolone carboxylic acid compound. Its activity against 740 bacterial isolates was determined. It inhibited 90% Escherichia coli, Klebsiella sp., Aeromonas sp., Enterobacter spp., Serratia spp., Proteus mirabilis, and Morganella morganii at less than or equal to 0.8 micrograms/ml [1]. Daily plasma theophylline concentrations were measured in 14 patients. The mean +/- s.d. theophylline concentrations increased from 8.5 +/- 2.8 micrograms ml-1 prior to enoxacin to a maximum of 21.7 +/- 7.8 micrograms ml-1 during coadministration [2]. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 226 °C |
|---|---|
| Molecular Formula | C15H17FN4O3.3/2H2O |
| Molecular Weight | 347.34 |
| PSA | 96.69000 |
| LogP | 0.99280 |
| Water Solubility | 1 M NaOH: soluble50mg/mL, clear, colorless to faintly yellow |
| Safety Phrases | 24/25 |
|---|