65899-73-2
| Name | Tioconazole |
|---|---|
| Synonyms |
Fungibacid
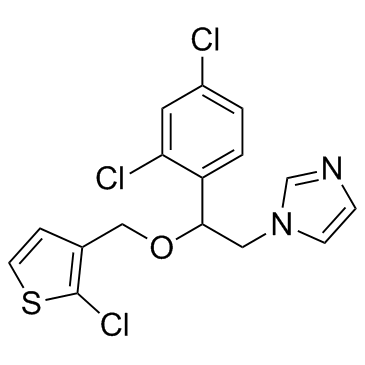
Vagistat 1-{2-[(2-chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl}imidazole Tioconazole Zoniden EINECS 265-973-8 1-[2-[(2-Chlorothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)-ethyl]imidazole 1-{2-[(2-chlorothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole 1-[2-[(2-Chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole 1-[2,4-Dichloro-b-[(2-chloro-3-thenyl)oxy]phenethyl]imidazole (±)-1-[2-[(2-Chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole MFCD00057276 1-{2-[(2-Chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole Trosyd |
| Description | Tioconazole is an antifungal medication.Target: AntifungalTioconazole is an antifungal medication of the Imidazole class used to treat infections caused by a fungus or yeast. Tioconazole topical (skin) preparations are also available for ringworm, jock itch, athlete's foot, and tinea versicolor or "sun fungus". Tioconazole interacts with 14-alpha demethylase, a cytochrome P-450 enzyme that converts lanosterol to ergosterol, an essential component of the yeast membrane. In this way, tioconazole inhibits ergosterol synthesis, resulting in increased cellular permeability [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 534.5±50.0 °C at 760 mmHg |
| Molecular Formula | C16H13Cl3N2OS |
| Molecular Weight | 387.711 |
| Flash Point | 277.0±30.1 °C |
| Exact Mass | 385.981415 |
| LogP | 5.05 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.654 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |
| RTECS | NI4480000 |