Tioconazole
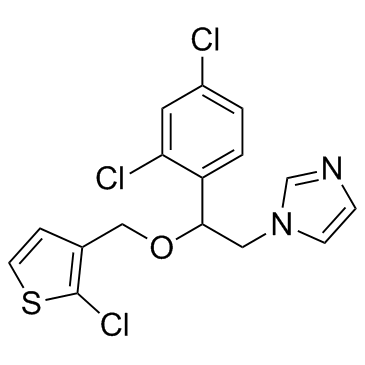
Tioconazole structure
|
Common Name | Tioconazole | ||
|---|---|---|---|---|
| CAS Number | 65899-73-2 | Molecular Weight | 387.711 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 534.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H13Cl3N2OS | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 277.0±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of TioconazoleTioconazole is an antifungal medication.Target: AntifungalTioconazole is an antifungal medication of the Imidazole class used to treat infections caused by a fungus or yeast. Tioconazole topical (skin) preparations are also available for ringworm, jock itch, athlete's foot, and tinea versicolor or "sun fungus". Tioconazole interacts with 14-alpha demethylase, a cytochrome P-450 enzyme that converts lanosterol to ergosterol, an essential component of the yeast membrane. In this way, tioconazole inhibits ergosterol synthesis, resulting in increased cellular permeability [1]. |
| Name | Tioconazole |
|---|---|
| Synonym | More Synonyms |
| Description | Tioconazole is an antifungal medication.Target: AntifungalTioconazole is an antifungal medication of the Imidazole class used to treat infections caused by a fungus or yeast. Tioconazole topical (skin) preparations are also available for ringworm, jock itch, athlete's foot, and tinea versicolor or "sun fungus". Tioconazole interacts with 14-alpha demethylase, a cytochrome P-450 enzyme that converts lanosterol to ergosterol, an essential component of the yeast membrane. In this way, tioconazole inhibits ergosterol synthesis, resulting in increased cellular permeability [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 534.5±50.0 °C at 760 mmHg |
| Molecular Formula | C16H13Cl3N2OS |
| Molecular Weight | 387.711 |
| Flash Point | 277.0±30.1 °C |
| Exact Mass | 385.981415 |
| LogP | 5.05 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.654 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |
| RTECS | NI4480000 |
|
Molecularly imprinted solid phase extraction of fluconazole from pharmaceutical formulations.
Talanta 134 , 1-7, (2015) This work encompasses a direct and coherent strategy to synthesise a molecularly imprinted polymer (MIP) capable of extracting fluconazole from its sample. The MIP was successfully prepared from metha... |
|
|
In vitro antimicrobial activity of plants used in traditional medicine in Gurage and Silti Zones, south central Ethiopia.
BMC Complement Altern. Med. 15 , 286, (2015) To overcome the escalating problems associated with infectious diseases and drug resistance, discovery of new antimicrobials is crucial. The present study aimed to carry out in vitro antimicrobial ana... |
|
|
A method to identify and validate mitochondrial modulators using mammalian cells and the worm C. elegans.
Sci. Rep. 4 , 5285, (2014) Mitochondria are semi-autonomous organelles regulated by a complex network of proteins that are vital for many cellular functions. Because mitochondrial modulators can impact many aspects of cellular ... |
| Fungibacid |
| Vagistat |
| 1-{2-[(2-chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl}imidazole |
| Tioconazole |
| Zoniden |
| EINECS 265-973-8 |
| 1-[2-[(2-Chlorothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)-ethyl]imidazole |
| 1-{2-[(2-chlorothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole |
| 1-[2-[(2-Chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole |
| 1-[2,4-Dichloro-b-[(2-chloro-3-thenyl)oxy]phenethyl]imidazole |
| (±)-1-[2-[(2-Chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole |
| MFCD00057276 |
| 1-{2-[(2-Chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole |
| Trosyd |
| 1H-Imidazole, 1-[2-[(2-chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]- |