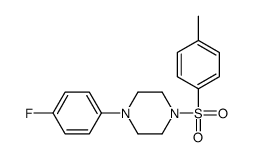
127625-29-0
| Name | Fananserin |
|---|---|
| Synonyms |
C23H24FN3O2S
fananserine UNII-38QJ762ET6 |
| Description | Fananserin (RP 62203) is an orally bioavailable, potent and selective 5-hydroxytryptamine2 (5-HT2) receptor antagonist, with a Ki of 0.37 nM for the rat 5-HT2A receptor. Fananserin also is a selective dopamine D4 receptor antagonist, with a Ki of 2.93 nM for the human dopamine D4 receptor[1]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 0.37 nM (5-HT2), 2.93 nM (human DA D4 receptor)[1] |
| In Vitro | Fananserin is relatively selective for 5-HT2 receptor, having lower affinity for the 5-HT1A receptor and very low affinity for the 5-HT3 receptor[1]. Fananserin displaces [3H]spiperone binding to recombinant human dopamine D 4 receptors with a Ki of 2.93 nM[1]. RP 62203 displays low to moderate affinity for α1-adrenoceptors, dopamine D2 receptors and histamine H 1 receptors[2]. |
| In Vivo | Fananserin displaces [125I]AMIK from 5-HT2 receptors with an IC50 of 0.21 nM in rat frontal cortex[2]. Fananserin shows moderate affinity for alpha 1-adrenoceptors in the rat thalamus (IC50 = 14 nM) and for histamine H1 receptors in the guinea-pig cerebellum (IC50 = 13 nM)[2]. Fananserin (0.5-4 mg/kg; p.o.) increases the duration of deep nonrapid eye movement (NREM) sleep at the expense of wakefulness in a dose-dependent manner[3]. Animal Model: Adult male Sprague Dawley rats (250-300 g)[3] Dosage: 0.5 mg/kg, 1 mg/kg,2 mg/kg, 4 mg/kg Administration: Oral administration Result: Increased the duration of deep nonrapid eye movement (NREM) sleep at the expense of wakefulness in a dose-dependent manner from 0.5 mg/kg. |
| References |
| Density | 1.331g/cm3 |
|---|---|
| Boiling Point | 641.1ºC at 760mmHg |
| Molecular Formula | C23H24FN3O2S |
| Molecular Weight | 425.51900 |
| Flash Point | 341.5ºC |
| Exact Mass | 425.15700 |
| PSA | 52.24000 |
| LogP | 4.84860 |
| Vapour Pressure | 2.48E-16mmHg at 25°C |
| Index of Refraction | 1.659 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
![2-(3-chloropropyl)-2H-naphth[1,8-cd]isothiazole 1,1-dioxide structure](https://image.chemsrc.com/caspic/386/127625-83-6.png)
![2H-naphth[1,8-cd]isothiazole 1,1-dioxide structure](https://image.chemsrc.com/caspic/455/603-72-5.png)