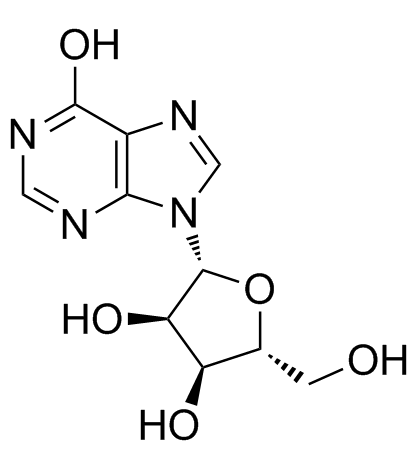
69655-05-6
| Name | didanosine |
|---|---|
| Synonyms |
9-[(2R,5S)-5-(hydroxyméthyl)tétrahydrofuran-2-yl]-1,9-dihydro-6H-purin-6-one
DDI Videx 9-[(2R,5S)-5-(Hydroxymethyl)tetrahydro-2-furanyl]-1,9-dihydro-6H-purin-6-one 9-[(2R,5S)-5-(Hydroxymethyl)tetrahydrofuran-2-yl]-9H-purin-6-ol MFCD00077728 VIDEX EC 2',3'-Dideoxyinosine 9-[(2R,5S)-5-(Hydroxymethyl)tetrahydrofuran-2-yl]-1,9-dihydro-6H-purin-6-one Dideoxyinosine Didanosine 9-[(2R,5S)-5-(Hydroxymethyl)tetrahydrofuran-2-yl]-1,9-dihydro-6H-purin-6-on didanosinum [INN_la] |
| Description | Didanosine(Videx) is a reverse transcriptase inhibitor with an IC50 of 0.49 μM.Target: NRTIs; HIVDidanosine is a dideoxynucleoside compound in which the 3'-hydroxy group on the sugar moiety has been replaced by a hydrogen. This modification prevents the formation of phosphodiester linkages which are needed for the completion of nucleic acid chains. Didanosine is a potent inhibitor of HIV replication, acting as a chain-terminator of viral DNA by binding to reverse transcriptase. Didanosine demonstrated linear pharmacokinetic behavior over the dose ranges of 0.4 to 16.5 mg/kg intravenously and 0.8 to 10.2 mg/kg orally. Bioavailability of didanosine when administered as a solution with an antacid was approximately 43% for doses from 0.8 to 10.2 mg/kg in patients with AIDS and advanced AIDS-related complex. Bioavailability of didanosine from the citrate-phosphate-buffered solution, the formulation currently used in phase II and expanded access studies, was comparable to the formulation used in the phase I trials [1]. ddI might be responsible for fulminant hepatitis in all three AIDS patients. This toxic effect may be added to the list of potential adverse events occurring during ddI therapy [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 531.2±60.0 °C at 760 mmHg |
| Melting Point | 193-195 °C |
| Molecular Formula | C10H12N4O3 |
| Molecular Weight | 236.227 |
| Flash Point | 275.0±32.9 °C |
| Exact Mass | 236.090942 |
| PSA | 93.03000 |
| LogP | -1.31 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.798 |
| Storage condition | 2-8°C |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | 1-5 g/100 mL at 21 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | C |
| Risk Phrases | R34 |
| Safety Phrases | S26-S27-S36/37/39-S45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | NM7460700 |
| HS Code | 2942000000 |
| Precursor 8 | |
|---|---|
| DownStream 2 | |
| HS Code | 2942000000 |
|---|
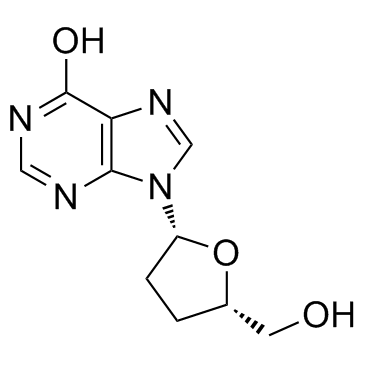
![N[SP]1[/SP],5'-O-dibenzyl-2',3'-dideoxyinosine structure](https://image.chemsrc.com/caspic/410/810694-79-2.png)
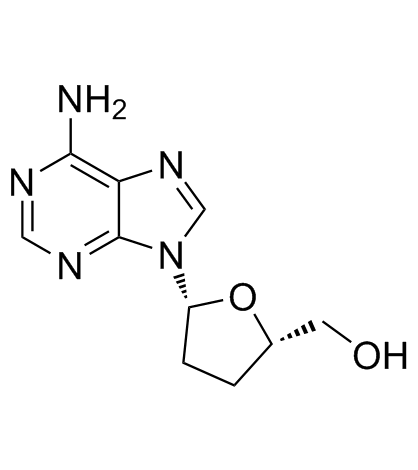
![9-[(2R,5S)-5-[[tert-butyl(dimethyl)silyl]oxymethyl]oxolan-2-yl]-3H-purin-6-one structure](https://image.chemsrc.com/caspic/418/177779-56-5.png)