6960-45-8
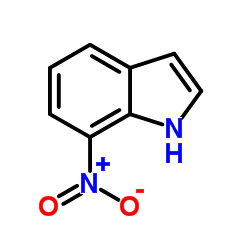
| Name | 7-Nitroindole-2-carboxylic acid |
|---|---|
| Synonyms |
MFCD00044720
7-Nitro-1H-indole-2-carboxylic acid EINECS 230-154-6 |
| Description | CRT0044876 is a potent and selective apurinic/apyrimidinic endonuclease 1 (APE1) inhibitor (IC50=~3 μM). CRT0044876 inhibits the AP endonuclease, 3′-phosphodiesterase and 3′-phosphatase activities of APE1, and is a specific inhibitor of the exonuclease III family of enzymes to which APE1 belongs. CRT0044876 potentiates the cytotoxicity of several DNA base-targeting compounds[1]. |
|---|---|
| Related Catalog | |
| In Vitro | A key step in BER is the processing of an apurinic/apyrimidinic (AP) site intermediate by an AP endonuclease. CRT0044876 has an IC50 for inhibition of APE1 of ∼3 μM and not only inhibits AP site cleavage catalyzed by purified APE1, but also cleavage directed by APE1 in a HeLa whole cell extract. CRT0044876 inhibits the 3′-phosphoglycolate diesterase activity of APE1 with an IC50 of ∼5 μM[1]. CRT0044876 inhibits both the exonuclease and AP endonuclease activities of exonuclease III, but shows no inhibitory activity towards endonuclease IV. CRT0044876 has minimal effects on BamHI restriction endonuclease or topoisomerase I even at CRT0044876 concentrations of 100 μM[1]. At non-toxic concentrations, CRT0044876 potentiates the cytotoxicity of several DNA damaging agents, which generate damage that is repaired in the BER pathway, including some currently-used anticancer drugs. The combination of MMS and CRT0044876 leads to a synergistic increase in the level of AP sites. Consistent with CRT0044876 being a specific BER inhibitor, a strong potentiation of hmdUrd cytotoxicity is seen in CRT0044876-treated cells (HT1080 cells)[1]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 520.8±30.0 °C at 760 mmHg |
| Melting Point | 260-261 °C |
| Molecular Formula | C9H6N2O4 |
| Molecular Weight | 206.155 |
| Flash Point | 268.8±24.6 °C |
| Exact Mass | 206.032761 |
| PSA | 98.91000 |
| LogP | 2.80 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.761 |
| Storage condition | room temp |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H319-H334 |
| Precautionary Statements | P261-P305 + P351 + P338-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R21/22;R36/37/38 |
| Safety Phrases | S36/37/39-S26-S22 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
|
~98% 
6960-45-8 |
| Literature: Purzer Pharmaceutical Co., Ltd. Patent: EP2366687 A2, 2011 ; Location in patent: Page/Page column 8; 10 ; |
|
~96% 
6960-45-8 |
| Literature: LG Life Sciences Ltd. Patent: US2010/210647 A1, 2010 ; Location in patent: Page/Page column 24 ; |
|
~% 
6960-45-8 |
| Literature: Journal of the American Chemical Society, , vol. 80, p. 4621 |
| Precursor 3 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |