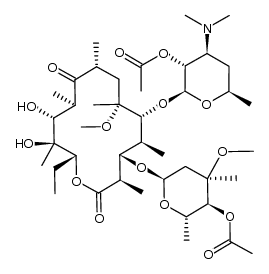
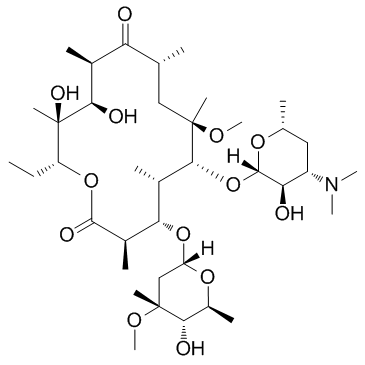
191114-48-4
| Name | Telithromycin |
|---|---|
| Synonyms |
KETEK
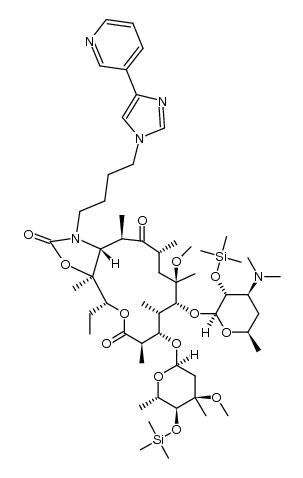
(3aS,4R,7R,9R,10R,11R,13R,15R,15aR)-4-ethyl-11-methoxy-3a,7,9,11,13,15-hexamethyl-2,6,8,14-tetraoxo-1-[4-(4-pyridin-3-yl-1H-imidazol-1-yl)butyl]tetradecahydro-2H-oxacyclotetradecino[4,3-d][1,3]oxazol-10-yl 3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranoside (3aS,4R,7R,9R,10R,11R,13R,15R,15aR)-10-{[(2S,3R,4S,6R)-4-(Dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-4-ethyl-11-methoxy-3a,7,9,11,13,15-hexamethyl-1-[4-(4-pyridin-3-yl-1H-imidazol-1-yl)butyl]octahydro-2H-oxacyclotetradecino[4,3-d][1,3]oxazol-2,6,8,14(1H,7H,9H)-tetron (3aS,4R,7R,9R,10R,11R,13R,15R,15aR)-4-Ethyl-11-methoxy-3a,7,9,11,13,15-hexamethyl-2,6,8,14-tetraoxo-1-{4-[4-(3-pyridinyl)-1H-imidazol-1-yl]butyl}tetradecahydro-2H-oxacyclotetradecino[4,3-d][1,3]oxazol ;-10-yl 3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranoside Telithromycin (3aS,4R,7R,9R,10R,11R,13R,15R,15aR)-4-Ethyl-11-methoxy-3a,7,9,11,13,15-hexamethyl-2,6,8,14-tetraoxo-1-{4-[4-(pyridin-3-yl)-1H-imidazol-1-yl]butyl}tetradecahydro-2H-oxacyclotetradecino[4,3-d][1,3]oxazol-10-yl 3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranoside (3aS,4R,7R,9R,10R,11R,13R,15R,15aR)-10-{[(2S,3R,4S,6R)-4-(diméthylamino)-3-hydroxy-6-méthyltétrahydro-2H-pyran-2-yl]oxy}-4-éthyl-11-méthoxy-3a,7,9,11,13,15-hexaméthyl-1-[4-(4-pyridin-3-yl-1H-imidazol-1-yl)butyl]octahydro-2H-oxacyclotétradécino[4,3-d][1,3]oxazole-2,6,8,14(1H,7H,9H)-tétrone (1R,2R,4R,6S,7R,8R,10R,13R,14S)-7-[(2S,3R,4S,6R)-4-Dimethylamino-3-hydroxy-6-methyloxan-2-yl]oxy-13-ethyl-6-methoxy-2,4,6,8,10,14-hexamethyl-17-[4-(4-pyridin-3-ylimidazol-1-yl)butyl]-12,15-dioxa-17-azabicyclo[12.3.0]heptadecane-3,9,11,16-tetrone (3aS,4R,7R,9R,10R,11R,13R,15R,15aR)-10-{[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-4-ethyl-11-methoxy-3a,7,9,11,13,15-hexamethyl-1-[4-(4-pyridin-3-yl-1H-imidazol-1-yl)butyl]octahydro-2H-oxacyclotetradecino[4,3-d][1,3]oxazole-2,6,8,14(1H,7H,9H)-tetrone MFCD04117983 3-De((2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl)oxy)-11,12-dideoxy-6-O-methyl-3-oxo-12,11-(oxycarbonyl((4-(4-(3-pyridinyl)-1H-imidazol-1-yl)butyl)imino))erythromycin |
| Description | Telithromycin(HMR3647) is a ketolide antibiotic to treat community acquired pneumonia of mild to moderate severity. Target: AntibacterialTelithromycin prevents bacteria from growing, by interfering with their protein synthesis. Telithromycin binds to the subunit 50S of the bacterial ribosome, and blocks the progression of the growing polypeptide chain. Telithromycin has over 10 times higher affinity to the subunit 50S than erythromycin. In addition, telithromycin strongly bind simultaneously to two domains of 23S RNA of the 50 S ribosomal subunit, where older macrolides bind strongly only to one domain and weakly to the second domain. Telithromycin can also inhibit the formation of ribosomal subunits 50S and 30S. From Wikipedia. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 966.2±65.0 °C at 760 mmHg |
| Melting Point | 176-188ºC |
| Molecular Formula | C43H65N5O10 |
| Molecular Weight | 812.004 |
| Flash Point | 538.2±34.3 °C |
| Exact Mass | 811.473145 |
| PSA | 171.85000 |
| LogP | 4.52 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.589 |
| Storage condition | -20?C Freezer |
|
~99% 
191114-48-4 |
| Literature: ALEMBIC LIMITED Patent: US2008/51352 A1, 2008 ; Location in patent: Page/Page column 14 ; |
|
~99% 
191114-48-4 |
| Literature: ALEMBIC LIMITED Patent: WO2008/23383 A1, 2008 ; Location in patent: Page/Page column 22 ; |
|
~% 
191114-48-4 |
| Literature: WO2005/105821 A2, ; Page/Page column 22 ; |
|
~49% 
191114-48-4 |
| Literature: Denis, Alexis; Agouridas, Constantin; Auger, Jean-Michel; Benedetti, Yannick; Bonnefoy, Alain; Bretin, Francois; Chantot, Jean-Francois; Dussarat, Arlette; Fromentin, Claude; Gouin D'Ambrieres, Solange; Lachaud, Sylvette; Laurin, Patrick; Le Martret, Odile; Loyau, Veronique; Tessot, Nicole; Pejac, Jean-Marie; Perron, Sebastien Bioorganic and Medicinal Chemistry Letters, 1999 , vol. 9, # 21 p. 3075 - 3080 |
|
~% 
191114-48-4 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 9, # 21 p. 3075 - 3080 |
|
~% 
191114-48-4 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 9, # 21 p. 3075 - 3080 |
|
~% 
191114-48-4 |
| Literature: Journal of Chemical Research, , vol. 37, # 2 p. 107 - 109 |
|
~% 
191114-48-4 |
| Literature: Journal of Chemical Research, , vol. 37, # 2 p. 107 - 109 |
|
~% 
191114-48-4 |
| Literature: Journal of Chemical Research, , vol. 37, # 2 p. 107 - 109 |
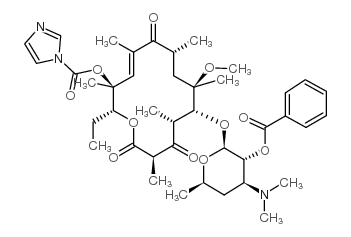
| Precursor 7 | |
|---|---|
| DownStream 0 | |