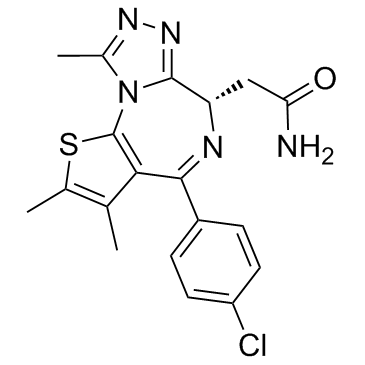
| Description |
CPI-203 is a novel potent, selective and cell permeable inhibitor of BET bromodomain, with an IC50 value of appr 37 nM (BRD4 α-screen assay).
|
| Related Catalog |
|
| Target |
IC50: 37 nM (BRD4)
|
| In Vitro |
CPI203 inhibits BRD4 in vitro and in cells, but does not affect BRD4 kinase activity in vitro[1]. CPI203 exerts a cytostatic effect in all the 9 MCL cell lines analyzed with GI50 ranging from 0.06 to 0.71 μM, with low cytotoxicity in normal PBMCs from healthy donors. Furthermore, lenalidomide and CPI203, by targeting IRF4 and MYC, efficiently activates the cell death program in MCL cells resistant to bortezomib[2].
|
| In Vivo |
BRD4 mediates CTD Ser2 phosphorylation in vivo[1]. In REC-1 tumor-bearing mice, the combination of lenalidomide with CPI203 (2.5 mg/kg, i.p.) synergistically augments the antitumoral properties of each single agent via the abrogation of MYC and IRF4 expression and the induction of apoptosis[2].
|
| Kinase Assay |
In vitro kinase assays with BRD4, PTEFb, and TFIIH are performed in 20 μL 50 mM Tris, pH 7.5, 5 mM DTT, 5 mM MnCl2, and 5 mM MgCl2 with 10 μCi γ32P ATP (6,000 Ci/mM) and/or 40 μM ATP where indicated. The kinase reactions are incubated for 1 h at 30°C, and then, the proteins are resolved by SDS/PAGE; the extent of phosphorylation is quantitated by a phosphorimager. For kinetic measurements, the quantitated values are plotted on a Lineweaver-Burke plot to calculate Vmax and Km values. When kinase inhibitors are used, appropriate dilutions of the inhibitor are added at the start of the kinase reaction. Mock-treated kinase reactions are treated with equivalent volumes of DMSO.
|
| Cell Assay |
MCL primary cells (1.5×105) and cell lines (4×104) are incubated as indicated with lenalidomide and/or PI203. MTT (3-(4,5-dimethylthiazolyl- 2)-2,5-diphenyltetrazolium bromide) reagent is added for 2-6 additional hours before spectrophotometric measurement. Each measurement is made in triplicate. Values are represented using untreated control cells as reference. The GI50 is calculated as the concentration that produced 50% growth inhibition. Combination indexes (CIs) are calculated by using the Calcusyn software version 2.0.
|
| Animal Admin |
CB17-severe combined immunodeficiency (SCID) mice are inoculated subcutaneously with 107 cells of the indicated MCL cell line, and monitored for tumor growth and vital parameters as previously described. For lenalidomide and lenalidomide-bortezomib dosing, mice are randomly assigned into cohorts of 3-4 mice each and receive by intraperitoneal injection a twice weekly dose of bortezomib (0.15 mg/kg), a daily dose of lenalidomide (50 mg/kg), the combination of lenalidomide and bortezomib, or an equal volume of vehicle. In the lenalidomide-CPI203 protocol, a total of 22 REC-1 tumor-bearing mice are randomly assigned to cohorts of 5-6 mice, receiving a twice daily intraperitoneal injection of 2.5 mg/kg CPI203, a daily intraperitoneal injection of 50 mg/kg lenalidomide, both agents or an equal volume of vehicle. Between 26 and 29 days post-inoculation, animals are killed according to institutional guidelines and tumor samples are subjected to immunohistochemical staining using primary antibodies against phospho-histone H3, cleaved caspase-3 (5A1E) and MYC (D84C12), IRF4 (M-17) and platelet endothelial cell adhesion molecule-1 (PECAM-1) (M20), CD19 (LE-CD19), Blimp-1 (clone Ros195G/G5), PAX5 (clone 24), CCL3 and CD38, as previously described. Preparations are evaluated using an Olympus DP70 microscope and Cell B Basic Imaging Software.
|
| References |
[1]. Devaiah BN, et al. BRD4 is an atypical kinase that phosphorylates serine2 of the RNA polymerase II carboxy-terminal domain. Proc Natl Acad Sci U S A. 2012 May 1;109(18):6927-32. [2]. Moros A, et al. Synergistic antitumor activity of lenalidomide with the BET bromodomain inhibitor CPI203 in bortezomib-resistant mantle cell lymphoma. Leukemia. 2014 Oct;28(10):2049-59.
|