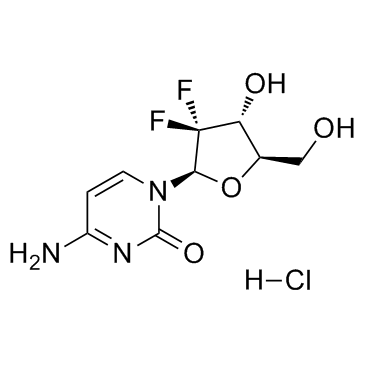
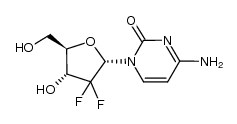
95058-81-4
| Name | gemcitabine |
|---|---|
| Synonyms |
4-AMINO-1-[(2R,4R,5R)-3,3-DIFLUORO-4-HYDROXY-5-(HYDROXYMETHYL)TETRAHYDROFURAN-2-YL]PYRIMIDIN-2-ONE
Gemcitabine GEMCITABINE, FREE BASE 2'-DESOXY-2',2'-DIFLUORCYTIDINE CYTIDINE, 2'-DEOXY-2',2'-DIFLUORO 3-DEOXY-2,2-DIFLUORO-D-RIBOFURANOSE-3,5-DIBENZOATE,0.97 4-amino-1-[3,3-difluoro-4-hydroxy-5- (hydroxymethyl) tetrahydrofuran-2-yl]- 1H-pyrimidin- 2-one GEMCITABINE (AS HCL) MFCD00869720 GEMCITABINE HCL 3-DEOXY-2,2-DIFLUORO-D-RIBOFURANOSE-3,5-DIBENZOATE 1-(2-deoxy-2,2-difluoro-β-D-erythropentofuranosyl)cytosine GEMCITABINE API 2'-DEOXY-2',2'-DIFLUOROCYTIDINE EINECS 619-100-6 |
| Description | Gemcitabine (NSC 613327;LY188011) is a DNA synthesis inhibitor which inhibits the growth of BxPC-3, Mia Paca-2, PANC-1, PL-45 and AsPC-1 cells with IC50s of 37.6, 42.9, 92.7, 89.3 and 131.4 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
DNA synthesis[1] |
| In Vitro | MTS assay demonstrates that Gemcitabine at 15 nM, indole-3-carbinol (I3C) at 50 μM and the combination does not affect hTERT-HPNE cell viability. However, treatment with Gemcitabine at 15 nM, I3C at 50 μM and the combination results in 31%, 19% and 72% cell death of BxPC-3 cells, respectively[1]. |
| In Vivo | Treatment of the LSL-KrasG12D/+; LSL-Trp53R172H; Pdx-1-Cre mice with either Gemcitabine (50 mg/kg, i.p.) or the combination DMAPT/Gemcitabine significantly increased the median survival time by more than 30 days compared to the placebo group (254.5 [P=0.015] or 255 days [P=0.018] vs. 217.5 days, respectively)[2]. Gemcitabine can be administered via endotracheal spray in rats without marked toxicity with a maximum tolerated dose of 4 mg/kg once a week for 9 weeks. The toxicity of Gemcitabine is lower via lung than oral administration at dosages of 2, 4, and 6 mg/kg[3]. |
| Cell Assay | Cells (the human pancreatic cell lines, Mia PaCa-2, BxPC-3, AsPC-1, PANC-1, PL-45, and normal pancreatic ductal epithelial cells, hTERT-HPNE cells) are seeded into 96-well plates (3000 cells/well) in triplicate. After overnight incubation, the medium is changed and cells are treated with I3C and/or NBMPR for 24 h. The medium is changed again and cells are cultured in medium containing different concentrations of Gemcitabine in the presence or absence of the same concentrations of I3C and/or NBMPR for 48 h. The cells are then subjected to CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS) as per the manufacturer's instructions. Absorbance at 490 nm is measured 2 h after the addition of 20 μL of MTS reagent/well[1]. |
| Animal Admin | Mice[2] At 1 month of age, LSL-KrasG12D/+; LSL-Trp53R172H; Pdx-1-Cre mice are randomized into treatment groups (placebo, DMAPT, Gemcitabine, DMAPT/Gemcitabine). Placebo (vehicle=hydroxylpropyl methylcellulose, 0.2% Tween 80 [HPMT]) and DMAPT (40 mg/kg body weight in HPMT) are administered by oral gastric lavage once daily. Gemcitabine (50 mg/kg body weight in PBS) is administered by intraperitoneal injection twice weekly. Mouse weight is monitored weekly. Treatment is continued until mice show signs of lethargy, abdominal distension or weight loss at which time they are sacrificed. Successful excision-recombination events are confirmed in the pancreata of mice by detecting the presence of a single LoxP site. Rats[3] The study is conducted in 80 female Wistar rats, with an initial weight of approximately 250 g. Animals are identified by ear mark and divided into groups as follows. Forty rats are divided into five groups of eight: four groups had lung delivery of Gemcitabine via endotracheal spray at doses of 2, 4, 6, and 8 mg/kg, respectively (groups LD2, LD4, LD6, LD8), and one group receive a spray administration of the 0.9% saline vehicle solution (group LDv). The remaining 40 rats are divided into five groups of eight: four groups have oral delivery of Gemcitabine via gavage at doses of 2, 4, 6, and 8 mg/kg, respectively (groups OD2, OD4, OD6, OD8), and one group receive an identical volume of 0.9% saline via gavage (group ODv). The protocol includes nine sessions separated by 1-week intervals. Between sessions, the animals are kept under standard laboratory conditions and housed by groups of four animals in each cage with litter and free access to pellet food and tap water. Cages are placed in a closed chamber connected to an aspiration system. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 468.0±55.0 °C at 760 mmHg |
| Melting Point | 168.64°C |
| Molecular Formula | C9H11F2N3O4 |
| Molecular Weight | 263.198 |
| Flash Point | 236.8±31.5 °C |
| Exact Mass | 263.071747 |
| PSA | 110.60000 |
| LogP | -1.24 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.652 |
|
Material Safety Data Sheet
Section1. Identification of the substance Product Name: Gemcitabine Synonyms: Section2. Hazards identification Harmful by inhalation, in contact with skin, and if swallowed. Section3. Composition/information on ingredients. Ingredient name:Gemcitabine CAS number:95058-81-4 Section4. First aid measures Skin contact:Immediately wash skin with copious amounts of water for at least 15 minutes while removing contaminated clothing and shoes. If irritation persists, seek medical attention. Eye contact:Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical attention. Inhalation:Remove to fresh air. In severe cases or if symptoms persist, seek medical attention. Ingestion:Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention. Section5. Fire fighting measures In the event of a fire involving this material, alone or in combination with other materials, use dry powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus should be worn. Section6. Accidental release measures Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national standards. Respiratory precaution:Wear approved mask/respirator Hand precaution:Wear suitable gloves/gauntlets Skin protection:Wear suitable protective clothing Eye protection:Wear suitable eye protection Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container for disposal. See section 12. Environmental precautions: Do not allow material to enter drains or water courses. Section7. Handling and storage Handling:This product should be handled only by, or under the close supervision of, those properly qualified in the handling and use of potentially hazardous chemicals, who should take into account the fire, health and chemical hazard data given on this sheet. Store in closed vessels, under −20◦C. Storage: Section8. Exposure Controls / Personal protection Engineering Controls: Use only in a chemical fume hood. Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles. General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse. Section9. Physical and chemical properties Appearance:Not specified Boiling point:No data No data Melting point: Flash point:No data Density:No data Molecular formula:C9H11F2N3O4 Molecular weight:263.2 Section10. Stability and reactivity Conditions to avoid: Heat, flames and sparks. Materials to avoid: Oxidizing agents. Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen fluoride. Section11. Toxicological information No data. Section12. Ecological information No data. Section13. Disposal consideration Arrange disposal as special waste, by licensed disposal company, in consultation with local waste disposal authority, in accordance with national and regional regulations. Section14. Transportation information Non-harzardous for air and ground transportation. Section15. Regulatory information No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302, or have known CAS numbers that exceed the threshold reporting levels established by SARA Title III, Section 313. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Hazard Codes | Xn,Xi |
|---|---|
| Risk Phrases | 21-36/38-46-62-63 |
| Safety Phrases | 25-26-36/37-53 |
| Precursor 5 | |
|---|---|
| DownStream 3 | |