364-98-7
| Name | diazoxide |
|---|---|
| Synonyms |
diazoxidum [INN_la]
7-chloro-3-methyl-4H-1λ<sup>6</sup>,2,4-benzothiadiazine 1,1-dioxide Hypertonalum Diazoxido 7-chloro-3-methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide Sodium dodecyl sulphate solution EINECS 206-668-1 Mutabase Proglycem Dizoxide Diazossido MFCD00078578 diazoxide Eudemine Proglicem 2H-1,2,4-Benzothiadiazine, 7-chloro-3-methyl-, 1,1-dioxide 7-chloro-3-methyl-1,2,4-benzothiadiazine-1,1-dioxide Hyperstat 7-Chloro-3-methyl-2H-1,2,4-benzothiadiazine 1,1-dioxide 7-chloro-3-méthyl-2H-1,2,4-benzothiadiazine-1,1-dioxyde 3-Methyl-7-chloro-1,2,4-benzothiadiazine 1,1-dioxide |
| Description | Diazoxide is an ATP-sensitive potassium channel activator ; can be used to treat hyperinsulinism. |
|---|---|
| Related Catalog | |
| In Vitro | Diazoxide has a number of physiological effects, including lowering the blood pressure and rectifying hypoglycemia. Diazoxide has powerful protective properties against cardiac ischemia[1].Diazoxide could protect NSC-34 neurons against the main sources of neurodegenerative damage. Diazoxide increases Nrf2 nuclear translocation in NSC-34 motoneurons and prevents endogenous oxidative damage[2]. |
| In Vivo | Diazoxide attenuates postresuscitation brain injury, protects mitochondrial function, inhibits brain cell apoptosis, and activates the PKC pathway by opening mitoKATP channels[3]. Treatment with diazoxide in wild-type mice decreases intraocular pressure (IOP) by 21.5±3.2% with an absolute IOP reduction of 3.9 ± 0.6 mm Hg[4]. |
| Cell Assay | Diazoxide is dissolved in DMSO to prepare 50 mM stock solution. NSC-34 cells are allowed to differentiate for 8 weeks under reduced serum conditions and then seeded in 24-well plates. Glutamate is dissolved in culture medium and added to cultures at concentration of 10 μM for 24 h. Cell treatment with 100 µM diazoxide starts 2 h before glutamate exposure. Cell viability is measured by the MTT assay[2]. |
| Animal Admin | Rats: Adult male Sprague-Dawley rats with induced cerebral ischemia (n=10 per group) receive an intraperitoneal injection of 0.1% DMSO (1 mL; vehicle group), diazoxide (10 mg/kg; DZ group), or diazoxide (10 mg/kg) plus 5-hydroxydecanoate (5 mg/kg; DZ + 5-HD group) 30 min after CPR. The control group (sham group, n=5) undergoes sham operation, without cardiac arrest. Mitochondrial respiratory control rate (RCR) is determined. Brain cell apoptosis is assessed using TUNEL staining. Expression of Bcl-2, Bax, and protein kinase C epsilon (PKCε) in the cerebral cortex is determined by Western blotting and immunohistochemistry[3]. Mouse: Diazoxide is prepared by diluting a 100 mM stock solution in 10% polyethoxylated castor oil in PBS. In C57BL/6 wild-type and Kir6.2(−/−) mice, a 5 μL drop of 5 mM diazoxide is topically administered to one eye of each mouse while the fellow control eye received vehicle (DMSO and 10% polyethoxylated castor oil in the same proportion as the treated eye). IOP is measured daily at 1 hour, 4 hours, and 23 hours following treatment. Treatment with diazoxide and vehicle is continued daily for 14 consecutive days[4]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 414.8±47.0 °C at 760 mmHg |
| Melting Point | >310°C |
| Molecular Formula | C8H7ClN2O2S |
| Molecular Weight | 230.671 |
| Flash Point | 204.6±29.3 °C |
| Exact Mass | 229.991669 |
| PSA | 66.91000 |
| LogP | 1.08 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.692 |
| Storage condition | Store at RT |
| Water Solubility | 0.1 M NaOH: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S22;S26;S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DK8185000 |
| HS Code | 2933990090 |
|
~90% 
364-98-7 |
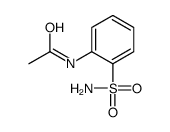
| Literature: Khelili, Smail; Faury, Gilles; Nicolle, Edwige; Verdetti, Jean; Leclerc, Gerard Medicinal Chemistry Research, 2003 , vol. 12, # 9 p. 457 - 470 |
|
~% 
364-98-7 |
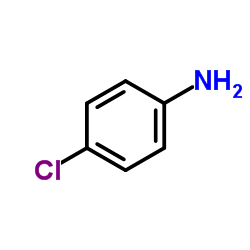
| Literature: Medicinal Chemistry Research, , vol. 12, # 9 p. 457 - 470 |
|
~% 
364-98-7 |
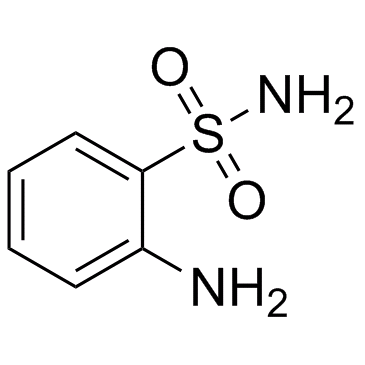
| Literature: Medicinal Chemistry Research, , vol. 12, # 9 p. 457 - 470 |
|
~% 
364-98-7 |
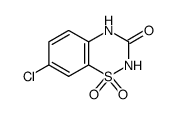
| Literature: Medicinal Chemistry Research, , vol. 12, # 9 p. 457 - 470 |
|
~% 
364-98-7 |
| Literature: Medicinal Chemistry Research, , vol. 12, # 9 p. 457 - 470 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |