5608-24-2
| Name | 2,2-bis(hydroxymethyl)-1-azabicyclo[2.2.2]octan-3-one |
|---|---|
| Synonyms |
Tocris-1862
prima-1 2,2-Bis(hydroxymethyl)quinuclidin-3-one 2,2-Bis-methylol-3-chinuclidinon MFCD04974196 p53 Reactivation and Induction of Massive Apoptosis 2,2-Bis(hydroxymethyl)-1-azabicyclo[2.2.2]octan-3-one |
| Description | PRIMA-1 is a mutant p53 reactivator, restores the sensitivity of TP53 mutant-type thyroid cancer cells to the histone methylation inhibitor 3-Deazaneplanocin A. |
|---|---|
| Related Catalog | |
| Target |
p53[1] |
| In Vitro | The cell lines are cultured in the presence of PRIMA-1 at 0-140 μM. The IC50s are 35, 40, 50, 50, 60, 70 and 75 μM for PANC-1, HEC-1-B, SUM149, AN 3CA, Ishikawa, Panc02 and MDA-MB-231 cells, respectively[2]. |
| In Vivo | PRIMA-1 (Prima-1) is a p53-modulating agent. 150 or 300 ppm PRIMA-1 significantly suppresses (P<0.0001) lung adenocarcinoma formation by 56% and 62%, respectively, after 17 weeks and 39% and 56%, respectively, after 34 weeks. Administration of 150 or 300 ppm PRIMA-1 significantly suppresses NNK-induced total lung adenocarcinoma formation by 57% or 62% (P<0.0001), respectively, after 17 weeks of exposure and by 39% or 56% (P<0.0001), respectively, after 34 weeks of exposure. As with administration of the lower (50 ppm) dose of CP-31398, administration of the lower (150 ppm) dose of PRIMA-1also slightly increases the number of NNK-induced lung adenomas[3]. |
| Cell Assay | A cell viability assay using yellow tetrazolium salt3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide or MTT is utilized to assess the effects of the p53 SMWC on growth of human carcinoma cell lines. Cells are plated in triplicate in 96-well plates at a density of 2.5×103 cells/well in 100 μL of complete medium. After 24hr incubation in a humidified 5% atmosphere at 37°C, the cells are treated with increasing concentrations of SMWC for an additional 24 hr period and analyzed for cell growth using the MTT assay. Stock solutions (10 mM) of CP-31398 and PRIMA-1 in PBS are diluted in PBS immediately prior to use. The assay is performed as follows: a 12 mM MTT stock solution is prepared by adding 1 mL of sterile PBS to 5 mg MTT and mixing by vortex or sonication until dissolved. Once prepared, the MTT solution is stored for four weeks at 4°C protected from light.A 500 mL SDS-HCl solution consisting of 0.01 M HCl, 10% propanol and 5 gm SDS is prepared by mixing the solution gently by inversion until the SDS dissolved.100 μL of cell culture medium is removed from each well and 10 μL of the 12 mM MTT stock solution added. A negative control consisting of 10 μL of the MTT stock solution added to 100 μL of medium is prepared. The plates are incubated at 37°C for 4hr followed by the addition of 100 μL of the SDS-HCl solution to each well and mixing thoroughly using a pipette. The absorbance of each sample is read at 570 nm in an ELISA plate reader. The inhibitory concentration (IC40) doses are determined using standard procedure[2]. |
| Animal Admin | Mice[3] Female A/J mice at 6 weeks of age are used. At 6 weeks of age, mice are fed control irradiated AIN-76A modified diet. At 7 weeks of age, the mice intended for carcinogen treatment receive a single dose of 10 mol (2.07 mg) NNK/mouse by intraperitoneal injection. All mice are weighed once every 2 weeks until termination of the study. Three weeks after NNK treatment, groups of mice (25 mice/group) are fed control AIN-76A or experimental diets containing 50 or 100 ppm CP-31398 or 150 or 300 ppm PRIMA-1 until termination. Mice are killed by CO2 asphyxiation followed by cervical dislocation after 17 weeks (10 mice/group) or 34 weeks (15 mice/group) of exposure to test agents. At the time of sacrifice, lungs are lavaged, perfused, and fixed in phosphate-buffered formalin, transferred within 2 days to 70% alcohol, and evaluated under a dissecting microscope for the number of tumors and tumor size. Tumors on the lung surface are enumerated by at least two experienced readers, blinded to sample identifiers, using a dissecting microscope. Tumor diameters are measured using Fisher brand digital calipers. |
| References |
[2]. Zhang Z, et al. Targeting cancer stem cells with p53 modulators. Oncotarget. 2016 Apr 8. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 353.7±17.0 °C at 760 mmHg |
| Molecular Formula | C9H15NO3 |
| Molecular Weight | 185.220 |
| Flash Point | 167.7±20.9 °C |
| Exact Mass | 185.105194 |
| PSA | 60.77000 |
| LogP | 0.70 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.582 |
| Storage condition | -20℃ |
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H334 |
| Precautionary Statements | P261-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R22;R43 |
| Safety Phrases | S36-S37 |
| RIDADR | NONH for all modes of transport |
|
~37% 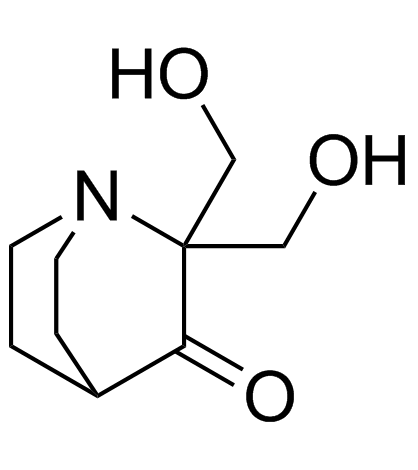
5608-24-2 |
| Literature: APREA AB Patent: WO2005/90341 A1, 2005 ; Location in patent: Page/Page column 12-13 ; |
|
~45% 
5608-24-2 |
| Literature: OHIO UNIVERSITY Patent: WO2007/62030 A2, 2007 ; Location in patent: Page/Page column 7 ; |
| Precursor 3 | |
|---|---|
| DownStream 4 | |

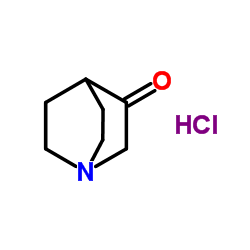
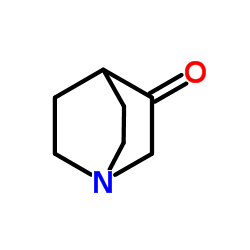
![(3-Oxo-1-azabicyclo[2.2.2]octane-2,2-diyl)bis(methylene) bis(2-me thylpropanoate) structure](https://image.chemsrc.com/caspic/462/865293-03-4.png)
![(3-Oxo-1-azabicyclo[2.2.2]octane-2,2-diyl)bis(methylene) dicyclob utanecarboxylate structure](https://image.chemsrc.com/caspic/386/865293-05-6.png)
![(3-Oxo-1-azabicyclo[2.2.2]octane-2,2-diyl)bis(methylene) dibenzoa te structure](https://image.chemsrc.com/caspic/348/865293-06-7.png)
![7,7-bis(hydroxymethyl)-1-azabicyclo[2.2.2]octan-8-ol structure](https://image.chemsrc.com/caspic/291/5291-31-6.png)