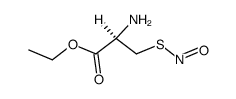
67776-06-1
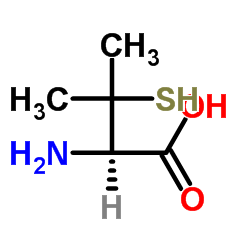
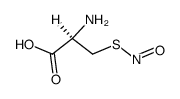
| Name | S-Nitroso-N-acetyl-DL-penicillamine |
|---|---|
| Synonyms |
MFCD00272624
N-Acetyl-3-(nitrososulfanyl)valine snap |
| Description | S-Nitroso-N-acetyl-DL-penicillamine (SNAP) is a nitric oxide donor and acts as a stable inhibitor of platelet aggregation[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | S-Nitroso-N-acetyl-DL-penicillamine (10 mM; 8 hours) induces toxicity of about 80% after 6 hours under normoxic conditions by releasing nitric oxide (NO)[1]. S-Nitroso-N-acetyl-DL-penicillamine has a half-time about 6 hours in in isolated rat ventricular myocytes[3]. S-Nitroso-N-acetyl-DL-penicillamine (100 µM; 30 minutes) causes sustained decrease in the basal pHi in isolated rat ventricular myocytes[3]. Cell Viability Assay[1] Cell Line: Rat liver sinusoidal endothelial cells Concentration: 2 mM, 5 mM, 10 mM Incubation Time: 2 hours, 4 hours, 6 hours, 8 hours Result: Exhibited cytotoxicity against cultivated endothelial cells. |
| In Vivo | SNAP (100μM, 300μM) causes small but significant increases of the electrically evoked [3H]-acetylcholine release in guinea-pig tracheal[4]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | 151ºC |
| Molecular Formula | C7H12N2O4S |
| Molecular Weight | 220.246 |
| Exact Mass | 220.051773 |
| PSA | 121.13000 |
| LogP | 1.13 |
| Index of Refraction | 1.560 |
| Storage condition | −20°C |
| Water Solubility | H2O: ≥2 mg/mL |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
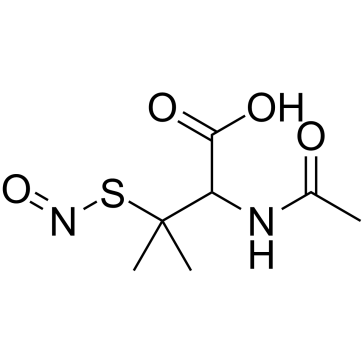
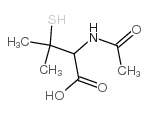
| Precursor 10 | |
|---|---|
| DownStream 7 | |
![Urea,N-(2-chloroethyl)-N-nitroso-N'-[(1-oxido-3-pyridinyl)methyl] structure](https://image.chemsrc.com/caspic/459/70015-86-0.png)