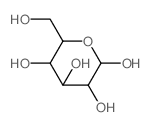
20229-56-5
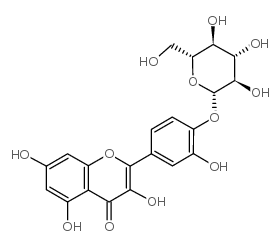
| Name | quercetin 4ʼ-O-β-D-glucopyranoside |
|---|---|
| Synonyms |
Spiraeosid
Quercetin 4'-b-D-glucopyranoside Quercetin 4'-O-glucoside Quercetin-4'-glucoside EINECS 243-614-6 Spiraein 3,5,7-trihydroxy-2-[3-hydroxy-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]chromen-4-one quercetin 4'-O-beta-D-glucopyranoside Spiraeoside |
| Description | Spiraeoside, an orally active natural compound, exerts antioxidant activity, inhibits reactive oxygen species (ROS) and malondialdehyde production. Spiraeoside possesses antiallergic, anti-inflammatory and antitumor activities[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Spiraeoside elevates HG stimulation-caused the decrease in the expression levels of p-Akt, nuclear Nrf2, and HO-1 in AC16 cells (the effects of Spiraeoside are reversedby LY294002)[1]. Spiraeoside protects AC16 cells against HG-induced oxidative stress, cell injury, and apoptosis[1]. Spiraeoside activates the PI3K/Akt/Nrf2 pathway in AC16 cells on exposure to HG[1]. Spiraeoside protects AC16 cells against HG-induced apoptosis through the PI3K/Akt/Nrf2 pathway[1]. Cell Viability Assay[1] Cell Line: AC16 cells. Concentration: 1, 5, 10, or 20 μM. Incubation Time: 0, 24, or 48 hours. Result: Inhibited AC16 cells viability (20 μM). |
| In Vivo | Spiraeoside (50 mg/kg, p.o.) shows ulcer preventive ability[2]. Animal Model: Male Wistar rats (6-8 weeks old)[2]. Dosage: 50 mg/kg. Administration: Oral gavage an hour before inducing the lesions. Result: Decreased severity of the formed lesions. |
| References |
| Density | 1.809g/cm3 |
|---|---|
| Boiling Point | 835.6ºC at 760mmHg |
| Melting Point | 209-211ºC |
| Molecular Formula | C21H20O12 |
| Molecular Weight | 464.37600 |
| Flash Point | 294.6ºC |
| Exact Mass | 464.09500 |
| PSA | 210.51000 |
| Vapour Pressure | 1.28E-29mmHg at 25°C |
| Index of Refraction | 1.774 |
| Storage condition | ?20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |
| Precursor 6 | |
|---|---|
| DownStream 2 | |


![2-(2,2-diphenylbenzo[d][1,3]dioxol-5-yl)-3,5,7-trihydroxy-4H-chroMen-4-one structure](https://image.chemsrc.com/caspic/359/357194-03-7.png)