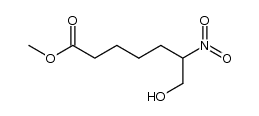
58962-34-8
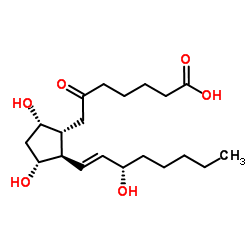
| Name | 6-oxo-prostaglandin F1α |
|---|---|
| Synonyms |
6-Oxoprostaglandin F1α
6-KETO-PROSTAGLANDIN F1ALPHA 6-OXO-9ALPHA,11ALPHA,15S-TRIHYDROXY-PROST-13E-EN-1-OIC ACID 6-oxo-prostaglandin F1alpha 6-Oxoprostaglandin F1alpha 6-KETOPROSTAGLANDIN F1A (9α,11α,13E,15S)-9,11,15-Trihydroxy-6-oxoprost-13-en-1-oic acid 6-Keto Prostaglandin 7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]cyclopentyl]-6-oxoheptanoic acid |
| Description | 6-keto Prostaglandin F1α is an endogenous metabolite present in Cerebrospinal_Fluid, Urine and Blood that can be used for the research of Meningitis, Rheumatoid Arthritis and Cardiopulmonary Resuscitation[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Endogenous metabolites is defined as those that are annotated by Kyoto Encyclopedia of Genes and Genomes as substrates or products of the ~1900 metabolic enzymes encoded in our genome. It is clear in the body of literature that there are documented toxic properties for many of these metabolites[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 575.3±50.0 °C at 760 mmHg |
| Molecular Formula | C20H34O6 |
| Molecular Weight | 370.480 |
| Flash Point | 315.8±26.6 °C |
| Exact Mass | 370.235535 |
| PSA | 115.06000 |
| LogP | 0.93 |
| Vapour Pressure | 0.0±3.6 mmHg at 25°C |
| Index of Refraction | 1.561 |
| Safety Phrases | S22-S24/25 |
|---|---|
| RIDADR | NONH for all modes of transport |
| Precursor 7 | |
|---|---|
| DownStream 0 | |